Harnessing Cooperative Multivalency in Thioguanine for Surface-Enhanced Raman Scattering (SERS)-Based Differentiation of Polyfunctional Analytes Differing by a Single Functional Group
Graphical Abstract
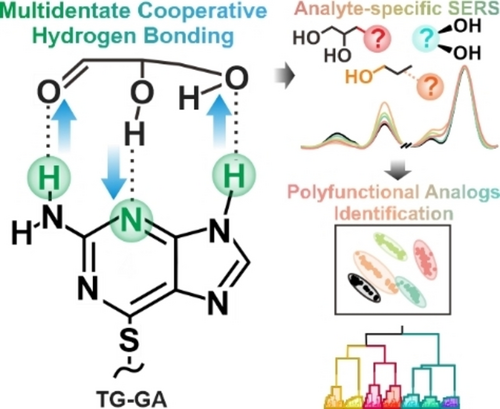
6-Thioguanine (TG), as a receptor capable of forming multidentate cooperative hydrogen bonding, enables the interaction with less-reactive functional groups and strengthens receptor-analyte complexation to achieve analyte-specific surface-enhanced Raman scattering (SERS) for accurate polyfunctional analog identification.
Abstract
Small-molecule receptors are increasingly employed to probe various functional groups for (bio)chemical analysis. However, differentiation of polyfunctional analogs sharing multiple functional groups remains challenging for conventional mono- and bidentate receptors because their insufficient number of binding sites limits interactions with the least reactive yet property-determining functional group. Herein, we introduce 6-thioguanine (TG) as a supramolecular receptor for unique tridentate receptor-analyte complexation, achieving ≥97 % identification accuracy among 16 polyfunctional analogs across three classes: glycerol derivatives, disubstituted propane, and vicinal diols. Crucially, we demonstrate distinct spectral changes induced by the tridentate interaction between TG's three anchoring points and all the analyte's functional groups, even the least reactive ones. Notably, hydrogen bond (H-bond) networks formed in the TG-analyte complexes demonstrate additive effects in binding strength originating from good bond linearity, cooperativity, and resonance, thus strengthening complexation events and amplifying the differences in spectral changes induced among analytes. It also enhances spectral consistency by selectively forming a sole configuration that is stronger than the respective analyte-analyte interaction. Finally, we achieve 95.4 % accuracy for multiplex identification of a mixture consisting of multiple polyfunctional analogs. We envisage that extension to other multidentate non-covalent interactions enables the development of interference-free small molecule-based sensors for various (bio)chemical analysis applications.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.