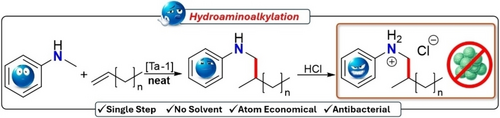
Hydroaminoalkylation for Amine Functionalization of Vinyl-Terminated Polyethylene Enables Direct Access to Responsive Functional Materials
Graphical Abstract
This paper presents a novel method for producing functionalized polyethylenes (PEs) via a 100 % atom economic tantalum-catalyzed hydroaminoalkylation of vinyl-terminated polyethylene (VTPE). Our approach addresses current synthetic challenges by enabling the efficient incorporation of amine functionalities into crystalline PEs in the melt phase. These functional materials have diverse potential applications and here we show that aminated PEs exhibit enhanced material properties, and pH responsive properties results in antibacterial PE films.
Abstract
While functionalized polyethylenes (PEs) exhibit valuable characteristics, the constraints of existing synthetic approaches limit the variety of readily incorporated functionality. New methods to generate functionalized PEs are required to afford new applications of this common material. We report 100 % atom economic tantalum-catalyzed hydroaminoalkylation of vinyl-terminated polyethylene (VTPE) as a method to produce amine-terminated PE. VTPEs with molecular weights between 2200–16800 g/mol are successfully aminated using solvent-free conditions. Our catalytic system is efficient for the installation of both aromatic and aliphatic amines, and can be carried out on multigram scale. The associating amine functional groups afford modified material properties, as measured by water contact angle, differential scanning calorimetry (DSC) and polymer rheology. The basic amine functionality offers the opportunity to convert inert PE into stimuli-responsive materials, such that the protonation of aminated PE affords the generation of functional antibacterial PE films.
As the most extensively utilized commodity plastic, PE significantly leads in global plastic production.1 PE has found extensive application in the manufacture of structural plastics and packaging materials, owing to its inertness, lightweight nature, cost-effectiveness, and exceptional formability.1, 2 The introduction of polar functional groups into PE offers broadened bulk properties that further enhance its application and utility.3 Moreover, recycling PE-based plastic waste is crucial for environmental sustainability.4 The decomposition of these plastics generates a variety of species, including alkene-containing polymers and oligomers.5 Should future advancements in the selective decomposition or separation of these alkene-containing species be realized,6 they could serve as valuable intermediates for hydrofunctionalization. Such developments have the potential to enable the production of higher-value chemicals, thereby imparting novel properties and expanding their fields of application.7 These forward-looking strategies for PE upcycling demand efficient PE functionalization methods.
The synthesis of functionalized PE involves two primary strategies: polymerization of functionalized alkene containing monomers8 and postpolymerization functionalization.9 The former is an intense area of research10 owing to the challenge of controlling the co-polymerization of functional monomers with simple alkenes.2c, 3, 11 Alternatively, postpolymerization functionalization is often plagued by side reactions, including cross-linking and chain scission, arising from radical or carbene/nitrene insertion mechanisms.12 Despite these common limitations, postpolymerization functionalization has been used for intentional PE crosslinking,13 PE oxidation,14 hydroxidation,15 halogenation,16 the installation of chromone,17 and sulfur containing groups.16, 18 Amination,19 among these functionalizations, stands out for its profound influence on PE properties, although, there are only a limited number of reports on this method (Scheme 1).

A, B & C) Previously reported amination of PE; D) Hydroaminoalkylation of vinyl-terminated polyethylene.
Amine functionalized materials display diverse properties owing to the inherent basicity, polarity, and nucleophilicity of amine groups. These features allow for stronger intermolecular interactions and heightened reactivity towards acidic and electrophilic groups.20 However, these same properties make the synthesis of amine functionalized PE challenging as catalyst deactivation and unwanted side reactions occur. To date limited strategies have been disclosed to access these materials. For example, in 2002, Bateman and Wu reported a non-catalytic direct amination of PE by insertion of a nitrene generated from 4-aminobenzenesulfonylazide.19a In 2013, Chase et al. reported an indirect postpolymerization amination of vinyl-terminated polyethylene (PE) using catalytic reductive amination routes (Scheme 1A).19b In 2017, Chen et al. reported a direct postpolymerization hydrazination method to indirectly install amines into PE (Scheme 1B).19c In 2023, Hartwig et al. outlined a complementary approach for direct postpolymerization amidation of PE, utilizing a copper C−H amidation catalytic system to install protected amine functionality (Scheme 1C).19d
These approaches all require multiple steps to access simple amine functionalized materials. We asked if we could expand our successful hydroaminoalkylation of amorphous, liquid vinyl-terminated polypropylene,21 to realize tantalum-catalyzed hydroaminoalkylation of crystalline VTPE as a one-step, atom-economic route for the efficient preparation of amine-terminated PE. As hydroaminoalkylation operates through a 2-electron, insertion, protonolysis route (see SI), unwanted radical-induced chain scission is avoided and desirable thermal and rheological properties of PE are retained. Finally, we show that even one single amine functionalization per PE chain results in modified physical properties and affords pH responsiveness that converts inert PE into ammonium functionalized PE with antibacterial properties.
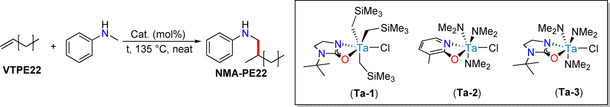
VTPE22 (Mn=2200 g/mol) and N-methylaniline (NMA) were chosen as reactants for the identification of optimized hydroaminoalkylation reaction conditions. The reaction progress could be monitored by ATR FT-IR spectroscopy. Successful amination was indicated by the complete disappearance of the terminal vinylic absorption at 1644 cm−1 and the simultaneous appearance of aromatic absorptions at 1506 and 1604 cm−1, as shown in Figure 1. The samples that showed complete conversion were subsequently analyzed by 1H NMR spectroscopy for yield determination (Figures S4 and S5). Complete conversion was validated through the absence of resonances corresponding to terminal vinyl and vinylidene groups at δ=4.98, 5.05, and 5.87 ppm respectively. Furthermore, distinctive spectral features of the NMA-PE22 product (Figure S6) were identified, including a triplet at 6.69 ppm for the aromatic ring, two diastereotopic doublets at 3.08 and 2.93 ppm for the N−CH2−C linkage, a broad singlet at 3.55 ppm for the NH proton, and a clear doublet at 1.01 ppm for the CH3 group, indicating regioselective formation of the branched structure. These observations collectively indicate the successful installation of NMA.
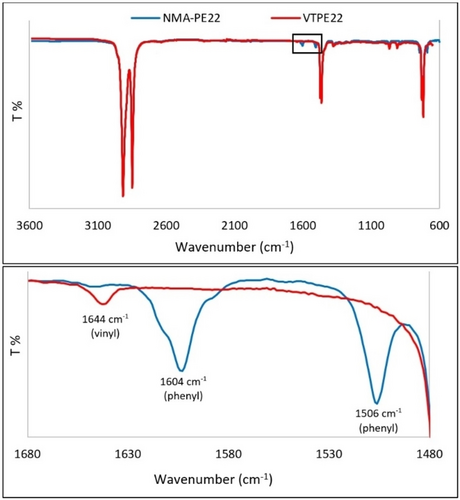
Stacked plot FT-IR spectra of VTPE22 and NMA-PE22.
No conversion was observed in the presence of homoleptic Ti(NMe2)4 and Ta(NMe2)5 complexes (Table 1, Entries 1 and 2, respectively). However, complete conversion was achieved in the presence of Ta(CH2SiMe3)3Cl2 (Entry 3). Reducing the loading of Ta(CH2SiMe3)3Cl2 from 5 to 3 mol % resulted in a notable drop in the yield to 15 % in an overnight reaction at 135 °C, where the polymer is in a melt phase and no solvent was required (Entry 4). However, Ta-1, a heteroleptic complex supported by a cyclic κ2-ureate,22 exhibited full conversion at only 3 mol % loading under neat reaction conditions (Entry 6). Lower catalyst loading (2 mol %) resulted in 62 % conversion (Entry 7). Careful reaction monitoring revealed that the neat reaction was complete within only 15 minutes, achieving a TOF of 133 h-1 (Entry 8). Ta-2,23 another potential pre-catalyst with reactive amido ligands rather than alkyl ligands, did not affect the hydroaminoalkylation of VTPE22 (Entry 9). Interestingly, the switch from the pyridonate ligand of Ta-2 to the cyclic amidate ligand (Ta-3) resulted in full conversion with 3 mol % after 1 h (Entries 10 and 11). However, a reduction in the reaction time to 30 min led to 37 % conversion when using Ta-3 (Entry 12). Consequently, Ta-1 was identified as the optimal candidate for conducting postpolymerization modification studies and scaled-up reactions (Table 2). Our Ta-1 catalytic system demonstrated efficiency in 5 g scale neat hydroaminoalkylation reactions of VTPE22 and VTPE25 (Mn=2500 g/mol) using NMA (Table 2, Entries 1 and 2), achieving over 90 % mass recovery by precipitating the product from a hot toluene solution by pouring into cold methanol. N-Methyl cyclohexylamine (NMCy), as a more challenging aliphatic amine substrate, required an overnight reaction (Entry 3).
|
||||
Entry |
Pre-catalyst |
Cat. (mol %)[b] |
Time[c] |
Yield[d] |
|---|---|---|---|---|
1 |
Ti(NMe2)4 |
5 |
O/N |
0 % |
2 |
Ta(NMe2)5 |
5 |
O/N |
0 % |
3 |
TaCl2(CH2SiMe3)3 |
5 |
O/N |
>99 % |
4 |
TaCl2(CH2SiMe3)3 |
3 |
O/N |
15 % |
5 |
Ta-1 |
5 |
O/N |
>99 % |
6 |
Ta-1 |
3 |
O/N |
>99 % |
7 |
Ta-1 |
2 |
O/N |
62 % |
8 |
Ta-1 |
3 |
15 min |
>99 % |
9 |
Ta-2 |
5 |
O/N |
0 % |
10 |
Ta-3 |
5 |
O/N |
>99 % |
11 |
Ta-3 |
3 |
60 min |
>99 % |
12 |
Ta-3 |
3 |
30 min |
37 % |
- [a] Conditions: VTPE22 (0.5 g, 0.223 mmol) and NMA (0.023 g, 0.223 mmol). [b] Catalyst loading as a mol % of amine. [c] O/N=overnight. [d] Yields were determined by 1H NMR analysis based on presence/absence of the characteristic resonances from the terminal vinyl and vinylidene of the reactant VTPE.
|
|||||
Entry |
VTPE[a] (g) |
Amine |
Solv.[b] |
Time[c] |
Conv./Yield[d] |
|---|---|---|---|---|---|
1 |
VTPE22 (5) |
NMA |
Neat |
0.5 h |
>99%/91% |
2 |
VTPE25 (5) |
NMA |
Neat |
0.5 h |
>99%/92% |
3 |
VTPE22 (3) |
NMCy |
Neat |
O/N |
>99%/91% |
4 |
VTPE90 (3) |
NMA |
Neat |
O/N |
>99%/94% |
5 |
VTPE140 (3) |
NMA |
Tol |
O/N |
>99%/90% |
6 |
VTPE168 (1) |
NMA |
Tol |
O/N |
>95%/93% |
7 |
VTPE22 (20) |
NMA |
Neat |
4 h |
>99%/91% |
8 |
VTPE140 (20) |
NMA |
Tol |
O/N |
>99%/90% |
- [a] Mn: (VTPE22=2236 g/mol, VTPE25=2507 g/mol, VTPE90=9024 g/mol, VTPE140=14065 g/mol, VTPE168=16798 g/mol). condition: VTPE (1 eq.) and amine (1 eq.). Catalyst loading as a mol% of amine. [b] Solv=solvent. [c] O/N=overnight. [d] Conv=conversions – conversions were determined by 1H NMR spectroscopic analysis based on presence/absence of the characteristic resonances from the terminal vinyl and vinylidene of the reactant VTPE/ Isolated yields of precipitated product.
Interestingly, materials incorporating aliphatic amines, even with their enhanced nucleophilicity/basicity, were not noted to display dramatically different properties (see below) and consequently further investigations focused on the more reactive NMA substrate. VTPE90 (Mn=9000 g/mol), a higher molecular weight VTPE, underwent full amination in a 3 g neat hydroaminoalkylation reaction with NMA (Entry 4). Notably, VTPE140 (Mn=14000 g/mol) and VTPE168 (Mn=16800 g/mol) were more viscous in the melt phase than the lower MW samples and required the addition of small amounts of toluene (3–5 mL) to allow proper stirring (Entries 5 and 6, respectively). Finally, as a further illustration that this transformation can be scaled, VTPE22 and VTPE140 were both evaluated on 20 g scale (Entries 7&8) and comparable isolated yields of aminated materials were obtained. GPC results revealed modest variation to PE molecular weights after amination, with retained polymer dispersity, indicating that the modification predominantly targeted specific sites without significantly altering the overall dispersity (see Figures S23 & S24 and Table S3).
In postpolymerization modification, residual catalyst can be incorporated into the resultant material.24 To quantify the amount of residual catalyst in our aminated polymers, both the vinyl-terminated and the aminated samples were analyzed by ICP-MS. The VTPE samples had Ta levels below or close to the detection limit (ppb) of the instrument, whereas the initially isolated aminated polymers exhibited Ta levels which ranged from 53 to 559 ppm and suffered from some spectral interference (Table S4). However, further purification of NMA-PE22, by dissolving in hot toluene and filtering through a glass frit, decreased the residual concentration by an order of magnitude and eliminated the spectral interference (Table S5).
A key research question was whether a single amine functionality would have any notable impacts on polymer physical properties. DSC results show that introducing one amine onto VTPEs causes a minor increase in the crystallization temperature (Tc, Figure 2). This observation is attributed to increased intermolecular associations between associating hydrogen bonding amine groups, as well as π–π stacking in the case of NMA, and consequent reduced chain mobility that demands slightly higher temperatures to initiate crystallization. The impact of PE molecular weight on the Tc is directly and linearly correlated (ΔTc=0.0001(Mn)+0.9081, R2=0.9928). Compared to their parent VTPE materials, changes of 2.6 °C, 1.9 °C, and 1.2 °C were observed for NMA-PE140, NMA-PE90, and NMA-PE22, respectively. The initiation temperature of crystallinity is also influenced by other parameters, such as the mobility of the dimers and, more particularly, molecular weight distribution (MWD). As MWD increases after amination, it can impact the initiation temperature of crystallinity. The most significant increase in MWD is observed in VTPE140, indicating a higher difference in Tc. In addition, while shorter chains are more susceptible to initial impacts from end-to-end interactions, longer chains can experience a more pronounced reduction in mobility due to dimer formation, potentially leading to a greater increase in Tc.25 Notably, the aliphatic NMCy substituent, with no π–π stacking, gave a slightly smaller change in Tc.9 These changes in Tc show that amination results in a decrease in the degree of crystallinity, which can be attributed to associations between amine groups. These associations hamper chain diffusion, thereby inhibiting the formation of an extended chain single crystal.26
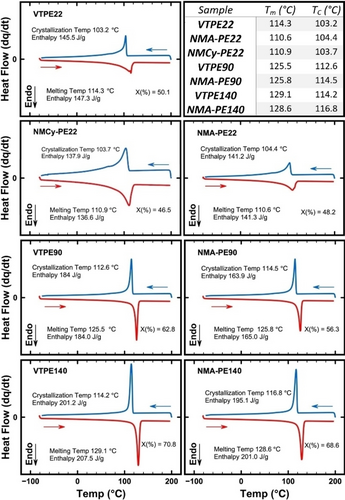
Melting temperature (Tm), crystallization temperature (Tc), enthalpy of fusion, and degree of crystallinity (X%) of VTPE22, VTPE90, VTPE140, NMA-PE22, NMCy-PE22, NMA-PE90 and NMA-PE140. Endo=endothermic.
Surface energy is a crucial factor for adhesion to paints and inks.27 To explore the influence of amine functionality on surface energy, the static water-contact angle of a film of NMA-PE90 was measured to be 85.1±1.5°, while that of VTPE90 was 91.8±1.5° (Figures S25 & S26). The reduced hydrophobicity of the NMA-PE90 film highlights the notable effect that even a small degree of amination (approx. 1 nitrogen for over 600 carbon atoms) can have on the surface energy of polymer films.19d, 28
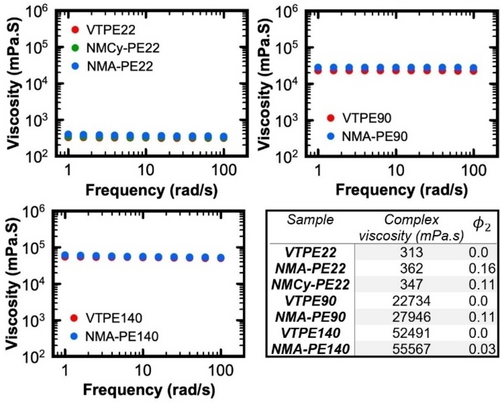
Complex viscosity versus frequency for VTPE22, VTPE90, VTPE140 and NMA-PE22, NMA-PE90 and NMA-PE140, obtained from frequency sweep measurement at constant shear strain in the linear viscoelastic regime at the constant temperature of 130 °C. ϕ2=dimer fraction.
Here and are the unimer and dimer viscosities, and ϕ1 and ϕ2 represent their corresponding volume fractions, respectively. In the dimer configuration, the dimer viscosity is calculated based on the scaling law represented by Equation 3,32 where M1 and M2 denote the molecular weights of unimers and dimers respectively, and α=1 if M2<Mc and α=3.4 if M2>Mc.
It is notable that NMA-PE22 is classified as an unentangled linear polymer below Mc while NMA-PE90 and NMA-PE140 are considered entangled linear systems. Consequently, NMA-PE140 exhibits the lowest dimer fraction compared to NMA-PE22 and NMA-PE90. This is attributed to diminished chain mobility within NMA-PE140, which consequently impedes optimization of associations with neighboring chains, thus limiting the formation of dimers. In addition, NMA-PE22 shows higher dimer fraction compared to NMCy-PE22, presumably due to its ability to engage in both hydrogen bonding and π–π interactions.
One of the differentiating features of amine functionalization of polyolefins is their responsive nature to pH. It is known that alkylated quaternary ammonium salts can be used as anti-microbial agents, such as the benzalkonium chlorides which display broad-spectrum antimicrobial properties.33 It functions by binding lipophilically and electrostatically at the water-membrane interface, disrupting the membrane and causing cellular leakage.34 However, research on simple protonated secondary ammonium salts as antimicrobials is limited, compared to their alkylated quaternary ammonium counterparts.35 Here we explored the possibility of using a simple protonation of the installed amine groups onto PE as a means of readily accessing anti-microbial plastics.
As a model system we investigated the hydroaminoalkylation of 1-octene using NMA in the presence of Ta-1 to give N-(2-methyloctyl)benzenamine (A-1). Subsequent protonation of A-1 with HCl produced ammonium salt Q-1 (Scheme S2). Antibacterial tests using this small molecule model system were conducted using the model strains of Gram-positive Staphylococcus aureus (ATCC 13709) and Gram-negative Escherichia coli (ATCC 25922). Upon incubation of the bacteria in the presence of Q-1 (1 % w/v) for 30 minutes at ambient conditions, total inactivation of both model strains of bacteria was observed.
Inspired by this result, NMA-PE22 and NMA-PE25 underwent protonation by treatment with HCl in ethereal solvents (see SI), resulting in the formation of Q-PE22 and Q-PE25 (Scheme S3 and S4, respectively). Antibacterial tests were conducted on VTPE22 and VTPE25 using model strains of S. Aureus and E. Coli. Under the same exposure conditions as Q-1, both S. Aureus and E. Coli displayed complete resistance to NMA-PE22 and NMA-PE25 (Tables S7 & S8). However, S. Aureus exhibited a 96 % reduction in viability when exposed to Q-PE22 and a 99.9 % reduction when exposed to Q-PE25 for 30 min (Table S7). In contrast, E. Coli remained completely resistant to both Q-PE22 and Q-PE25 (Table S8). These results are not unexpected, as Gram-negative bacteria typically display reduced susceptibility to antimicrobial agents due to the presence of their protective outer membrane.34 Importantly, it is known that Ta lacks inherent antibacterial properties,36 which clarifies that the antimicrobial activity observed for both Q-PE22 and Q-PE25 cannot be attributed to the ppm levels of Ta impurity in aminated samples.
In conclusion, our study establishes Ta-1 catalyzed hydroaminoalkylation as an efficient, direct method for the postpolymerization amination of VTPE. This method can be used on crystalline VTPEs of variable molecular weight and we have shown that these transformations can be scaled to 20 g and carried out under neat reaction conditions. Our thermal, surface energy, and rheological findings underscore the notable impact of even a small degree of amination on polymer properties. Such amine functionality offers opportunities for further reactivity, ion binding or as shown here, pH responsiveness. By using a simple HCl treatment of the readily accessed amine-terminated PE, inert crystalline polymers were converted to antibacterial films. This research unveils a transformative method for directly accessing responsive functional materials from simple polyolefins by postpolymerization amination in the melt phase.
Acknowledgments
The authors thank the NSERC Alliance Program for funding. SSS thanks NSERC for a doctoral scholarship. This work was funded in-part through the Canada Research Chairs program to LLS.
Conflict of Interests
The catalyst used for this work is patented and licensed to A2O Advanced Materials Inc. L. L. Schafer is a founder of A2O Advanced Materials Inc.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.