A Dibenzo[g,p]chrysene-Based Organic Semiconductor with Small Exciton Binding Energy via Molecular Aggregation
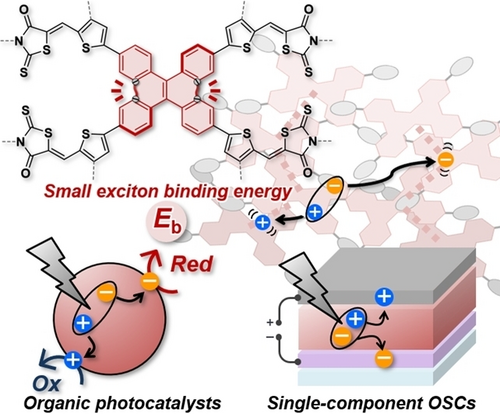
Graphical Abstract
Exciton binding energy (Eb) is recognized as a key physical parameter for efficient charge-carrier generation in opto-electronic devices and organic photocatalysts (OPCs). The correlation between Eb and aggregation is important to achieve a small level of Eb in the solid state. Here, the influence of aggregation behavior on Eb and device/OPC functions were revealed by using two organic semiconductors with different aggregation behaviors.
Abstract
Exciton binding energy (Eb) is understood as the energy required to dissociate an exciton in free-charge carriers, and is known to be an important parameter in determining the performance of organic opto-electronic devices. However, the development of a molecular design to achieve a small level of Eb in the solid state continues to lag behind. Here, to investigate the relationship between aggregation and Eb, star-shaped π-conjugated compounds DBC-RD and TPE-RD were developed using dibenzo[g,p]chrysene (DBC) and tetraphenylethylene (TPE). Theoretical calculations and physical measurements in solution showed no apparent differences between DBC-RD and TPE-RD, indicating that these molecules possess similar properties on a single-molecule level. By contrast, pristine films incorporating these molecules showed significantly different levels of electron affinity, ionization potential, and optical gap. Also, DBC-RD had a smaller Eb value of 0.24 eV compared with that of TPE-RD (0.42 eV). However, these molecules showed similar Eb values under dispersed conditions, which suggested that the decreased Eb of DBC-RD in pristine film is induced by molecular aggregation. By comparison with TPE-RD, DBC-RD showed superior performances in single-component organic solar cells and organic photocatalysts. These results indicate that a molecular design suitable for aggregation is important to decrease the Eb in films.
Introduction
In equation (1), e is the elementary charge, ϵ0 is the vacuum dielectric constant, ϵr is the relative dielectric constant, and R is the average electron-hole distance. Most organic semiconductor materials possess Eb values that range from 0.3 to 0.6 eV.7 Since these values are much larger than thermal energy at room temperature (0.03 eV), such a large value for Eb is understood to cause energy loss and charge recombination in OSCs and OPCs.8 Thus, the establishment of a molecular design that could achieve small values for Eb in the solid state is an important objective in the development of high-performance opto-electronic devices.9
Several experimental studies have revealed that extending π-conjugated skeletons7b, 9a, 10 and introducing polar functional groups7b, 9a, 11 are parts of an effective molecular design to lower the Eb based on the tuning of dielectric responses at the single-molecule level (Figure 1a). For example, in π-conjugated compound Y6-4O, the alkyl groups of representative nonfullerene acceptor Y6 are replaced by an oligoethylene oxide side chain, which reduces the value of Eb and increases the external quantum efficiency (EQE) to 1.4 % in single-component (SC) OSCs (Figure S1 in the Supporting Information).11a On the other hand, theoretical studies suggest that assembled structures in the solid state significantly affect the Eb of organic semiconductors, and that aggregation and dimensional packing are beneficial for effective electronic polarization (Figure 1b).7c, 9, 12 There is no corresponding experimental study, however, that has yet focused on the relationship between the aggregated states of organic semiconductor molecules and Eb.
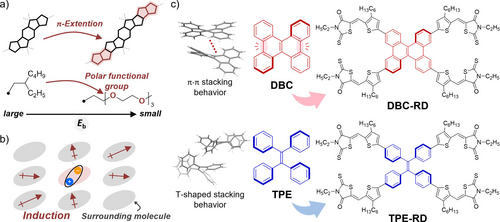
(a) Molecular design to produce a small Eb on a single-molecule level. (b) Induction effect from surrounding molecules in a solid state. (c) Chemical structures and packing motifs of central units.
To investigate the effect that the aggregation of organic semiconducting materials exerts on Eb in the solid state, we decided to utilize two-dimensional dibenzo[g,p]chrysene (DBC) and tetraphenylethylene (TPE) as key frameworks (Figure 1c), because we previously found that a two-dimensional extension of phenylenevinylene oligomers with delocalized frontier molecular orbitals effectively increases electronic polarization.13 DBC and TPE are known to form different π-π stacking behaviors. The π-conjugated planes of DBC are stacked in a single-crystal state,14 whereas TPE exhibits a T-shaped stacking behavior.15 To tune the frontier molecular orbital energy level to accommodate the function of an organic semiconductor, we introduced electron-accepting terminal units of 3-ethylrhodanine (RD)16 and spacer units of 3-hexylthiophene, and thus produced the target compounds DBC-RD and TPE-RD. In this report, we describe our investigation into the effect that molecular aggregation in the solid state exerts on the Eb and on the opto-electronic functions in SC-OSCs and OPCs.
Results and Discussion
Theoretical Investigation
Density functional theory (DFT) calculations at the M06-2X level were performed to investigate the optimized molecular geometries, frontier orbitals, and polarizability (α) of DBC-RD and TPE-RD. To include polarization and diffuse function, Pople's 6–31+G(d,p) basis set was employed. Note that all alkyl groups of these compounds were replaced by methyl groups to reduce the computational load (denoted as DBC-RD(m) and
TPE-RD(m) for clarity). As shown in Figure 2a, both DBC-RD(m) and TPE-RD(m) possess non-planar three-dimensional (3D) molecular structures, and the geometrical features reflect the central skeletons of DBC and TPE.14, 15 While TPE-RD(m) possesses a propeller-shaped conformation with the benzene ring twisted at a dihedral angle of approximately 45° against the molecular center, DBC-RD(m) takes a saddle-shaped conformation with a relatively small twist angle of 24°. As shown in Figure 2b, the highest occupied molecular orbitals (HOMOs) and the lowest unoccupied molecular orbitals (LUMOs) were delocalized over the entire π-conjugated backbones for both DBC-RD(m) and TPE-RD(m). The estimated HOMO/LUMO energy levels of DBC-RD(m) and TPE-RD(m) were −5.44/−2.96 and −5.45/−2.92 eV, respectively. The α values of DBC-RD(m) and TPE-RD(m) were estimated to be 1,382 and 1,389 Bohr3, respectively. These calculated results indicate that on a molecular level electronic properties such as the distributions of frontier molecular orbitals, energy levels, and α values, have little influence on the steric structures of DBC-RD(m) and TPE-RD(m).
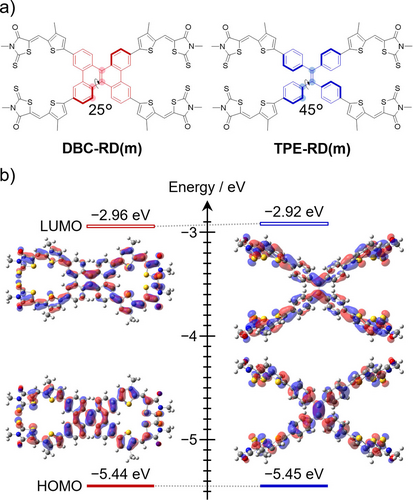
(a) Optimized molecular structures and (b) frontier molecular orbitals and their energy levels for DBC-RD(m) and TPE-RD(m).
Synthesis and Thermal Properties
The synthetic routes to DBC-RD and TPE-RD are shown in Scheme 1. Compound 2 was obtained via Suzuki–Miyaura cross-coupling between 117 and boronated thiophene derivative 318 following a deprotecting reaction using hydrochloric acid. Compound 5 was synthesized via a cross-coupling reaction between 419 and 5-bromo-3-hexylthiophene-2-carbaldehyde20. Finally, Knoevenagel condensation reactions using 2 and 5 with 3-ethylrhodanine afforded DBC-RD and TPE-RD, respectively. The synthetic details and characterization data are supplied in the SI.
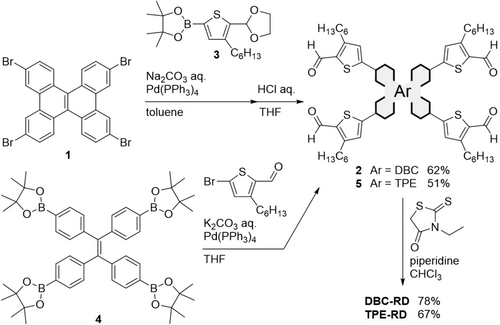
Synthetic routes to DBC-RD and TPE-RD.
The thermal properties of DBC-RD and TPE-RD were investigated by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). TGA profiles showed a 5 %-weight-loss temperature (Td) of 328 and 359 °C for DBC-RD and TPE-RD, respectively, which indicates that these compounds possess sufficient thermal stabilities for use as organic semiconducting materials (Figure S2a). In the DSC measurements (Figure S2b), melting points (Tm) were observed at 136 and 131 °C for DBC-RD and TPE-RD, respectively.
To investigate the aggregation behavior in solutions, we measured the variable-concentration 1H NMR spectra in CDCl3.
As shown in Figure 3, at 20 °C a 10 mg mL−1 concentration of DBC-RD showed broad signals in the aromatic region, along with two sharp singlet peaks at 7.4 and 7.9 ppm. All the broad signals were assigned to the protons on the DBC framework, while two sharp peaks were assigned to the protons on the thiophene rings and vinyl groups, respectively. The broad signals were down-field shifted and sharpened when the concentration was decreased from 10 to 1 mg mL−1. This concentration dependence could be attributed to the aggregation behavior of the DBC framework in the solution. In contrast to DBC-RD, TPE-RD showed no apparent concentration dependence even at a relatively high concentration of 10 mg mL−1 (Figure S3b), which indicates a lack of aggregation for TPE-RD.
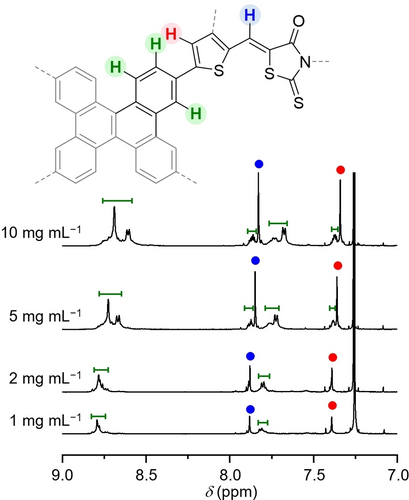
Variable-concentration 1H NMR spectra for DBC-RD in CDCl3. The entire spectra are given in Figure S3a.
Molecular Properties in Solution
UV/Vis absorption and emission spectra of DBC-RD and TPE-RD in CHCl3 solutions are shown in Figures 4 and S4, and the photophysical data are summarized in Table 1. The maximum absorption wavelengths ( ) of DBC-RD and TPE-RD were observed at 458 and 452 nm, respectively. DBC-RD and TPE-RD showed onset absorption ( ) at 528 and 514 nm, respectively, which corresponds to the HOMO–LUMO transitions. These compounds showed fluorescence with maximum emission wavelengths at 570 and 577 nm, respectively (Figure S4).
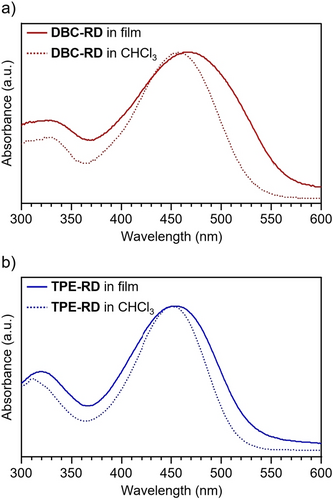
UV/Vis absorption spectra for (a) DBC-RD and (b) TPE-RD.
Compound |
(nm) |
(nm) |
(nm) |
(nm) |
Egopt (eV) |
EHOMO[a] (eV) |
ELUMO[b] (eV) |
EA[c] (eV) |
IP[d] (eV) |
Eb[e] (eV) |
|---|---|---|---|---|---|---|---|---|---|---|
DBC-RD |
458 |
528 |
464 |
563 |
2.20 |
−5.52 |
−3.21 |
3.41 |
5.85 |
0.24 |
TPE-RD |
452 |
514 |
454 |
529 |
2.34 |
−5.53 |
−3.14 |
3.25 |
6.01 |
0.42 |
- [a] EHOMO=−(Eox1+4.8). [b] ELUMO=−(Ered1+4.8). [c] Determined by LEIPS. [d] Determined by PYS. [e] Determined by the equation (2).
To investigate the electrochemical properties, differential pulse voltammetry (DPV) measurements of DBC-RD and TPE-RD were conducted in o-dichlorobenzene:acetonitrile (5 : 1) solutions containing 0.1 M tetra-n-butylammonium hexafluorophosphate (TBAPF6) as a supporting electrolyte (Figure S5). The redox potentials were referenced against ferrocene/ferrocenium (Fc/Fc+) as an internal standard. The first oxidation (Eox1)/reduction (Ered1) peaks of DBC-RD and TPE-RD appeared at 0.73/−1.59 and 0.72/−1.66 V, respectively. Based on the assumption that (Fc/Fc+) would be less than 4.8 eV from the vacuum level,21 the HOMO/LUMO energy levels of DBC-RD and TPE-RD were calculated to be −5.52/−3.21 and −5.53/−3.14 eV, respectively. These results indicate that the central frameworks of both TPE and DBC have little influence on the photophysical and electrochemical properties on a single-molecule level.
Properties in Film
We measured the UV/Vis absorption spectra of DBC-RD and TPE-RD in pristine films. As shown in Figure 4, the onset wavelength ( ) of the TPE-RD film showed a red-shift of 15 nm in a solution-spectrum comparison. In the case of DBC-RD, the corresponding red-shift of the onset wavelength was increased to 35 nm, indicating the presence of more distinct intermolecular electronic interactions for DBC-RD. X-ray diffraction (XRD) measurements of the DBC-RD and TPE-RD films showed a bulge between 20 and 25 degrees, which originated from intermolecular π-π stacking (Figure S6). The diffraction maximum of the bulge for DBC-RD (2θ=21.8°, 4.08 Å) was slightly larger than that for TPE-RD (21.3°, 4.17 Å), indicating that DBC-RD tends to form a closed π-π stacking structure. These results imply that the influence of intermolecular interactions in the solid state is more prominent for DBC-RD.
To gain insight into the molecular arrangements of DBC-RD and TPE-RD, we conducted molecular dynamics (MD) simulations using the Gromacs 2023.5.22 The computational details are provided in the SI. As shown in Figure S7, DBC-RD and TPE-RD showed different molecular stacking statistics. Based on the existence probabilities summarized in Table S1, DBC-RD tends to adopt more π-π stacked structures compared with that produced by TPE-RD. These simulated results support the experimental results of thin films.
In equation (2), the Egtrans, Egopt, IP, and EA are defined as the transport gap, optical gap, ionization potential, and electron affinity, respectively. To obtain the EA and IP of DBC-RD and TPE-RD in films, low-energy inverse photoemission spectroscopy (LEIPS)23 and photoelectron yield spectroscopy (PYS)24 measurements were conducted (Figure 5a,b).7 Based on the onset of the spectra shown in Figure 5, the EA/IP values were determined to be 3.41/5.85 eV for DBC-RD and 3.25/6.01 eV for TPE-RD. The Egopt values estimated from the onset of the absorption spectra were determined to be 2.20 eV for DBC-RD and 2.34 eV for TPE-RD. Based on these values, the Eb values for DBC-RD and TPE-RD were determined to be 0.24 and 0.42 eV, respectively (Figure 5c). The Egopt values were also estimated from the intersection of the absorption and fluorescence spectra (Figure S4),7a, 25 and the Eb values based on these Egopt were also determined (Table S2). As expected, DBC-RD showed a smaller Eb value than that of TPE-RD, which also was smaller than the values reported for the semiconductor materials of Y6 (0.34 eV) and ITIC (0.31 eV).7a
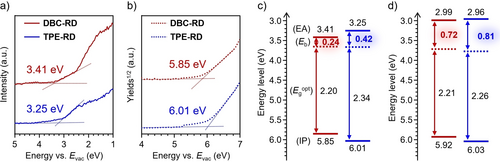
(a) LEIPS and (b) PYS spectra of DBC-RD (red) and TPE-RD (blue) in films. Energy diagrams for (c) pristine and (d) C8-POSS-dispersed films.
To investigate the influence that aggregation has on the Eb in films, we also measured the Eb of DBC-RD and TPE-RD under dispersed conditions (Ebdilute) in an octyl-substituted silsesquioxane (C8-POSS) matrix (Figure S8). To prevent the formation of aggregation, we selected a low concentration of 3 wt % against C8-POSS.26 The results of LEIPS, PYS, and UV/Vis spectra are shown in Figure S9, and a summary of the energy diagram appears in Figure 5d. Based on these results, the Ebdilute values of the DBC-RD and TPE-RD films under diluted conditions were 0.72 and 0.81 eV, respectively. The difference in Ebdilute between DBC-RD and TPE-RD was small relative to the difference between them in Eb. We assumed that the absence of intermolecular interactions led to the appearance of molecular-level properties that resulted in the similar Ebdilute values, which is in agreement with the calculated results that showed identical α values for DBC-RD and TPE-RD. The Ebdilute value of DBC-RD was larger than its aggregated Eb value (0.24 eV), which indicates that the low Eb value of the DBC-RD pristine film should be attributed to the formation of molecular aggregation in the solid state.
SC-OSC Characteristics
In order to evaluate the photon-to-electron conversion function for DBC-RD and TPE-RD, SC-OSC devices were constructed using a configuration of ITO/ZnO/DBC-RD or TPE-RD/MnO3/Ag. Details of the fabrication procedure and the SC-OSC performance are provided in the Supporting Information (Tables S3–5). The current density (J)-voltage (V) curves of the devices under an air mass 1.5 G of solar irradiation (100 mW cm−2) are shown in Figure 6a, and the corresponding photovoltaics parameters are summarized in Table 2. Although a negligible degree of photocurrent was observed in TPE-RD-based SC-OSCs, the DBC-RD-based SC-OSCs showed typical OSC characteristics. The highest power conversion efficiency (PCE) of the DBC-RD-based SC-OSC was 0.14 % with a short-circuit current density (Jsc) of 0.87 mA cm−2, an open-circuit voltage (Voc) of 0.33 V, and a fill factor (FF) of 49 %. The film morphologies of SC-OSCs were investigated via atomic force microscopy (AFM). As shown in Figure S10, both the DBC-RD and TPE-RD films formed smooth surfaces and morphologies with average roughness values of 1.2 and 0.3 nm, respectively. EQE measurement returned a maximum EQE value (EQEmax) of 6.43 % at 450 nm for the DBC-RD-based device (Figure 6b). These Jsc and EQEmax values are larger than those reported for Y6 (Jsc=0.316 mA cm−2, EQEmax=1.0 %) or Y6-4O (0.476 mA cm−2, EQEmax=1.4 %),11a which indicates that DBC-RD possesses a relatively high level of photon-to-current conversion characteristics.
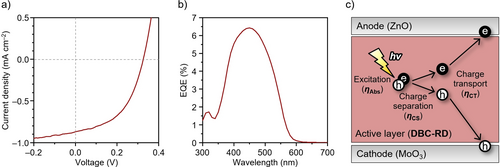
(a) J–V curve; (b) EQE spectrum for a DBC-RD-based SC-OSC; (c) Photon-to-current conversion process in a SC-OSC.
Compound |
JSC (mA cm−2) |
VOC (V) |
FF (%) |
PCE[a] (%) |
EQEmax (%) |
ϵr |
t1/2 (min) |
k[c] (min−1) |
|---|---|---|---|---|---|---|---|---|
DBC-RD |
0.87 |
0.33 |
49 |
0.14 (0.08±0.03) |
6.4 |
3.36 |
3.8 |
0.221 |
TPE-RD |
0.04 |
0.03 |
25 |
0.00 |
n.a.[b] |
3.00 |
15.2 |
0.039 |
- [a] The average for 5 devices; standard deviation is provided in parenthesis. [b] This data were not available because of limited photocurrent from the device. [c] Estimated by a pseudo first-order reaction kinetics.31
The overall photon-to-current conversion process in the photo-active layer can be understood by considering the following three factors (Figure 6c): incident photon absorption (ηAbs), the charge separation from excitons to free carriers (ηCS), and the charge transport (ηCT).11a In order to investigate the relevance of these factors to the Jsc and EQEmax values of SC-OSCs, we compared the transmittance spectra of pristine films using either DBC-RD or TPE-RD. As shown in Figure S11, these spectra showed no significant difference in either transmittance or spectral shape, which implies that the ηAbs is not the dominant factor in SC-OSC characteristics. To evaluate the ηCT, flash-photolysis time-resolved microwave conductivity (FP-TRMC) measurements were performed.27 As shown in Figure S12, DBC-RD and TPE-RD exhibited similar maximum values for transient photoconductivities (φΣμmax) of 1.0×10−5 and 1.1×10−5 cm2 V−1 s−1, respectively. This result indicates that the ηCT of the DBC-RD and TPE-ED films was virtually on the same level.
Considering these results, the ηCS should be the origin of the high Jsc and EQEmax values for the DBC-RD-based SC-OSC. To support this result, we measured the ϵr values for DBC-RD and TPE-RD. The electrical impedance measurements were conducted using a multilayer device composed of glass/ITO/ZnO/DBC-RD or TPE-RD/MoO3/Al.16b, 28 Figure 7 shows the Nyquist plots and an assumed equivalent circuit. Based on the estimated capacitances and film thickness (Table S4), the ϵr values for DBC-RD and TPE-RD were estimated at 3.36 and 3.00, respectively. Based on the inversely proportional relationship (eq. 1), the larger ϵr value of DBC-RD would be consistent with a smaller Eb value. From these results, we concluded that the high Jsc and EQEmax of DBC-RD originated from the ηCS, which was facilitated by the small Eb value of DBC-RD.
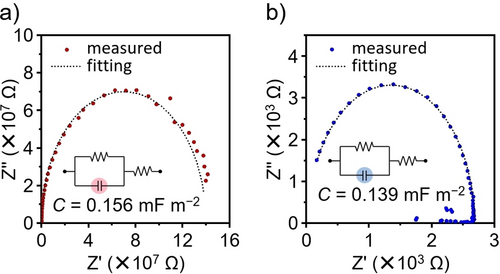
Nyquist plots and assumed equivalent circuits for (a) DBC-RD and (b) TPE-RD.
OPC Characteristics
To utilize photocarrier generation behavior in heterogeneous photoredox reactions, we investigated the use of DBC-RD and TPE-RD solid particles as photocatalysts. The photocatalytic activities were evaluated by using the oxidative decomposition of 1,1′,3,3,3′,3′-hexamethylindotricarbocyanine iodide (HITCI) as an indicator (Figure S13).29, 30 Figure S14 shows the time-dependent absorption spectra under the illumination of a light-emitting diode with a wavelength of 465 nm, and time-dependent decay of HITCI based on these absorption spectra appear in Figures 8 and S15. Under blank conditions, negligible quenching was observed for up to 30 minutes. On the other hand, UV-quenching was observed in the presence of DBC-RD and TPE-RD particulates, and DBC-RD showed a significantly higher level of catalytic activity. The results for quenching the rate constant (k)31 and half-life time (t1/2) are summarized in Table 2. We attribute the higher photocatalytic activity of DBC-RD to the enhanced charge-separation process, facilitated by the small value of Eb.
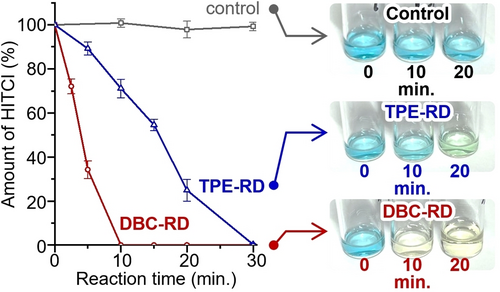
(Left) Time-dependent decay of HITCI monitored at 760 nm under an average of 4 measurements. Error bars indicate the standard deviation. (Right) Photo images for the reactions of DBC-RD and TPE-RD.
Conclusions
In conclusion, to investigate the influence that the aggregation of organic semiconductor molecules exerts on Eb in the solid state, we designed and synthesized two star-shaped π-conjugated compounds: DBC-RD and TPE-RD. Concentration-dependent NMR measurements showed an aggregation behavior for DBC-RD. Photophysical and electrochemical measurements in solutions as well as calculated results showed that both compounds possess similar physical properties on a single-molecule level. By contrast, these compounds showed different values for EA, IP, and Egopt in pristine films, and thus DBC-RD led to a smaller Eb value compared with that of TPE-RD. On the other hand, thin films of DBC-RD and TPE-RD under dispersed conditions showed similar Ebdilute values. These results indicate that the smaller Eb of DBC-RD in pristine films was caused by strong intermolecular interactions. DBC-RD-based SC-OSCs showed typical photovoltaic responses with a maximum PCE of 0.14 %. Analyses of the photon-to-current conversion process indicate that the ηCS should be the key process that determines the photovoltaic characteristics. Moreover, DBC-RD exhibited higher photocatalytic activity in heterogeneous OPC reactions compared with that of TPE-RD. These results suggest that aggregation behavior in the solid state is important for a reduction in the Eb of organic semiconductors, which will contribute to the development of high-performance opto-electronic devices and organic photocatalysts.
Supporting Information
The data that support the findings of this study are available in the supplementary material of this article.
Acknowledgments
This work was supported by JSPS KAKENHI (20H02814, 20H05841, 20KK0123, 20 K15352, JP20H05836, 22H01938, 23H02064, and 24H00482), JST-CREST (JPMJCR20R1), JST-Mirai (JPMJMI22I1), JST−A-step (JPMJSF23B3), JST SPRING (JPMJSP2138), the Mitsubishi Foundation (202310004), and “Dynamic Alliance for Open Innovation Bridging Human, Environmental and Materials” from The Ministry of Education, Culture, Sports, Science and Technology, Japan. We are thankful to Nanotechnology Open Facilities, Osaka University (JPMXP09S21OS0010) for PYS measurements. Thanks are extended to the CAC, SANKEN, for assistance in obtaining elemental analyses and high-resolution mass spectra.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.