Enantioconvergent Cross-Nucleophile Coupling: Copper-Catalyzed Deborylative Cyanation
Graphical Abstract
We report a general deborylative cyanation protocol representative of an enantioconvergent cross-nucleophile coupling platform. Mechanistic study suggests the formation of prochiral alkyl radicals via aniline-assisted single-electron activation of organoboron species. Electronic structure analysis of the reactive CuII(CN)2 complex indicates a significant amount of ligand-based radical density.
Abstract
Organoboron compounds are widely utilized in organic synthesis for their diverse reactivity, modular preparation, and stability compared to other classes of organometallic reagents. While organoboron species are commonly employed as nucleophiles in cross-coupling reactions, their potential as racemic building blocks in enantioconvergent transformations remains largely untapped. Herein, we demonstrate the direct utilization of alkylboronic pinacol esters in intermolecular enantioconvergent transformations. Specifically, this work describes the development and mechanistic study of an enantioconvergent deborylative cyanation enabled by Cu catalysis. This method imparts a high degree of enantioselectivity and tolerates a wide range of common functional groups and heterocycles. The reaction is proposed to proceed through a radical-relay mechanism. Aniline-assisted homolysis of the carbon–boron bond results in prochiral alkyl radicals that are functionalized by in situ generated Cu(II)(CN)2 species in an enantioselective fashion. The Cu(II)(CN)2 intermediate was characterized by electron paramagnetic resonance (EPR) spectroscopy, and its electronic structure was probed using density functional theory (DFT) calculations. Computational studies were carried out to corroborate the proposed radical-relay mechanism.
Introduction
Organoboron compounds represent one of the most versatile and widely utilized classes of reagents in organic synthesis.1 Unlike most types of organometallic reagents, common organoboron species, such as alkylboronic pinacol esters (APEs), are non-toxic, highly functional-group-tolerant, and bench-stable under ambient conditions.1b The synthesis of organoboron compounds, particularly APEs, is highly modular.2 The iterative homologation of APEs can rapidly and selectively introduce structural complexity into simple starting materials.3 The unique stability and reactivity of α-borylcarbanions render them easily accessible intermediates in the construction of alkyl frameworks.4 However, contrary to their aryl and alkenyl-organoboron congeners, the application of APEs in cross-coupling reactions is often complicated by sluggish transmetalation as well as common side reactions such as protodeboronation.5 In particular, their uses as racemic building blocks in enantioconvergent intermolecular cross-coupling reactions would be highly desirable yet remain underexplored.6 The three existing relevant protocols rely upon the photocatalytic cleavage of C−B bonds in APE-derived alkyl trifluoroborates. Following the initial report by Molander and Kozlowski,7 strategies to access enantiomerically enriched N-benzylic heterocycles and α-aryl ketones were recently disclosed by Reisman8 and Dong,9 respectively (Figure 1A). Given their stability and accessibility, a general mechanistic approach to directly engage APEs in enantioconvergent cross-coupling reactions would offer a convenient retrosynthetic disconnection, expediting access to functionalized alkyl frameworks. In this regard, we disclose a new Cu-catalyzed cross-nucleophile coupling platform for the asymmetric synthesis of alkyl nitriles.
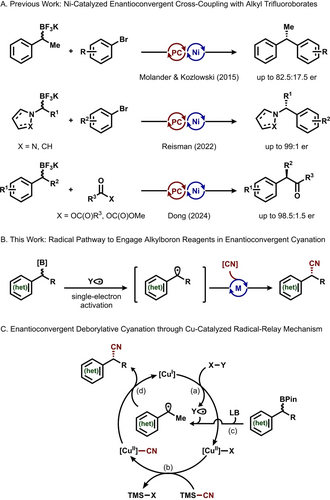
(A) Ni-catalyzed cross-coupling reactions facilitated by photochemical activation as the only examples to use alkylboron reagents in enantioconvergent transformations. (B) Engaging alkylboron reagents in enantioconvergent cyanation through single-electron activation. (C) Proposed Cu-catalytic cycle for enantioconvergent cross-nucleophile cyanation of alkylboron reagents through a radical relay mechanism.
Enantiomerically enriched alkyl nitriles are featured in a wide range of bioactive molecules.10 In the context of drug discovery, the mild electrophilicity of nitrile groups enables the selective covalent inhibition of various protein targets.11 While a number of asymmetric cyanation protocols have been reported recently,12 the direct conversion of easily accessible APEs into alkyl nitriles in an enantioconvergent fashion would offer a powerful synthetic route to these valuable substructures. However, the synthetic modularity of APEs enabled by their empty p-orbital comes at the cost of a high oxidation potential compared to their trifluoroborate derivatives.13 Correspondingly, we envisioned a Lewis base activator could facilitate the in situ formation of a tetracoordinate adduct to effectively modulate the single electron reactivity of APEs and assist their conversion into prochiral radicals in a stereoablative fashion.14 A chiral transition metal catalyst could then be used to intercept the carbon radical to install various functional groups and establish an oxidative cross-coupling platform (Figure 1B). Seminal work by Liu and Stahl, along with a series of subsequent studies, demonstrated that Cu(II)(CN)2 complexes are highly effective at stereoselectively transferring cyano groups to alkyl radicals.12b, 15 Therefore, we hypothesized that the combination of a chiral Cu catalyst with an external oxidant could enable the cross-nucleophile coupling between alkylboronic ester and cyanide through a radical-relay mechanism.16
In the proposed transformation (Figure 1C), a single-electron transfer between the Cu(I) catalyst and oxidant generates a Cu(II) intermediate and a reactive radical species Y⋅ (step a). Transmetalation with the nucleophilic cyanide affords the Cu(II)(CN)2 complex (step b), while the single-electron activation of alkylboronic ester generates the prochiral alkyl radical (step c). Aided by a chiral ligand, a cyano group transfer mediated by the Cu(II)(CN)2 intermediate furnishes the nitrile product in an enantioselective manner (step d). We envisioned that a major challenge to this approach involves the judicious choice of compatible chemical oxidant and Lewis basic activator to balance the rates of boronic ester activation and Cu(II)(CN)2 generation. Herein, we disclose the successful implementation of this mechanistic strategy for an enantioconvergent cross-nucleophile cyanation protocol and the corresponding mechanistic studies to guide our future expansion of this reactivity platform.
Results and Discussion
Reaction Development and Optimization
We began our investigation by examining the cross-nucleophile coupling between alkylboronic pinacol ester 1 a and trimethylsilyl cyanide (TMSCN). We examined various copper sources, chiral bis(oxazoline) (BOX) ligands, chemical oxidants, and solvents (see Supporting Information, Table S1 for full optimization details). We identified a combination of CuOAc and ligand L1 as the optimal catalyst mixture, which provided cyanation product 2 a with the highest yield and enantioselectivity (entries 1–4, Table 1).
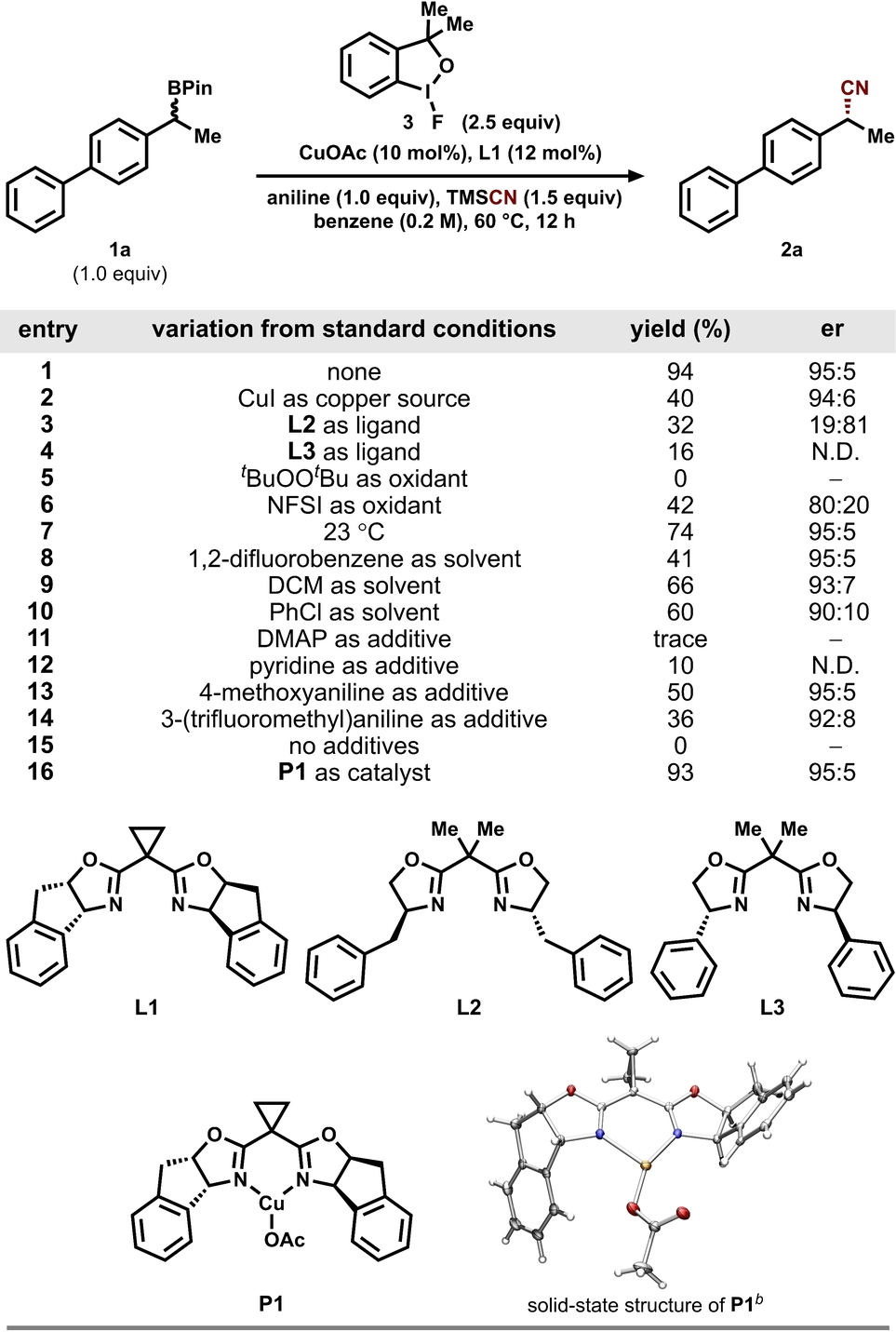
- aReaction conditions unless otherwise noted: 2-(1-([1,1′-biphenyl]-4-yl)ethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (1 a, 0.10 mmol), trimethylsilyl cyanide (TMSCN, 0.15 mmol), aniline (0.10 mmol), specified catalyst mixture, 1-fluoro-3,3-dimethyl-1,3-dihydro-1 l3-benzo[d][1,2]iodoxole (3, 0.25 mmol), and benzene (0.2 M); reaction yields were determined by 1H NMR spectroscopy of the crude reaction mixture using 1,1,2,2-tetrachloroethane as an internal standard (see the Supporting Information for details). The enantiomeric ratio (er) of product 2 a was determined by chiral high-performance liquid chromatography (HPLC) analysis. bSolid-state molecular structure of P1 with thermal ellipsoids at 50 % probability level. Color Scheme: Cu, gold; N, blue; O, red; C, gray; H, white.
Among the oxidants tested, commercially available fluorinated hypervalent iodine(III) reagent 3 afforded the highest yield compared to other commonly employed oxidants in radical relay reactions (entries 5–6 and Table S1).16 We anticipate that the Lewis basic additive is vital in modulating the single-electron reactivity of alkylboron reagents.14b, 17 A range of substituted anilines and neutral N-lone-pair donors such as DMAP and pyridine were tested as the Lewis basic additive.14a The highest yield and enantioselectivity were obtained with a stoichiometric amount of aniline (entries 11–15). To simplify the reaction protocol, a precatalyst (L1)CuOAc (P1) was prepared and utilized in subsequent experiments. The use of P1 afforded 2 a in similar yield and enantioselectivity to that obtained using a mixture of CuOAc and L1 (entry 16).
Substrate Scope of the Enantioconvergent Deborylative Cyanation Protocol
With the optimized reaction conditions identified for the formation of 2 a, we assessed the functional group tolerance and scope of compatible alkylboronic pinacol esters (Table 2). The reaction proceeds effectively with substrates bearing both electron-donating and –withdrawing functional groups. A range of heterocycles commonly found in pharmaceuticals were also well-tolerated, including pyridine (2 g, 2 t), quinoline (2 l), thiazole (2 h, 2 j), benzoxazole (2 c, 2 x), dibenzothiophene (2 b), pyrrole (2 q), indazole (2 w), benzothiophene (2 i), benzothiazole (2 k, 2 u), dibenzofuran (2 e), and oxetane (2 r).18 The absolute configuration of the major enantiomer was determined by obtaining the solid-state structures of products 2 b and 2 u (Figure S6).
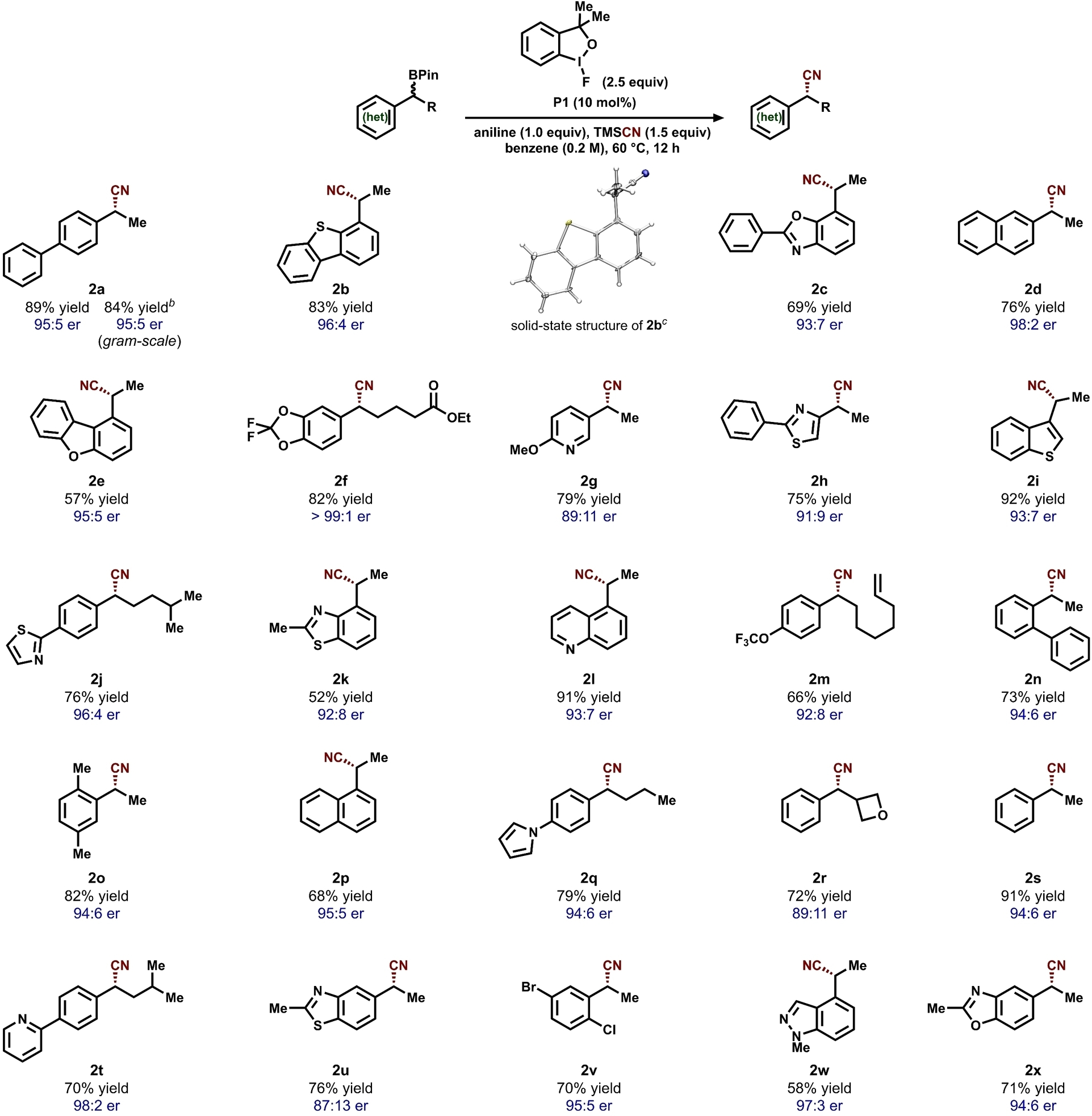
- aAll yields were obtained using 0.50 mmol of alkylboronic pinacol ester substrates unless otherwise noted. Enantiomeric ratio (er) was determined by chiral HPLC analysis. bReaction carried out on a 5.00 mmol scale of substrate 1 a. cSolid-state molecular structure of 2 b with thermal ellipsoids at 50 % probability level. Color scheme: S, yellow; N, blue; C, gray; H, white.
Benzylic boronic pinacol esters featuring ortho- (2 n, 2 o, 2 v), meta- (2 f, 2 o, 2 v), and para- (2 a, 2 j, 2 m) substituents are all suitable coupling partners. Cyanation of substrates containing electron-deficient aza-heterocycles (2 g, 2 t, 2 w) proceeds effectively with no detectable side products resulting from radical addition. The protocol is compatible with weak allylic (2 m) and benzylic C−H bonds (2 o), offering complementary reactivity to C−H functionalization strategies.15a, 19 To further highlight the synthetic utility of the enantioconvergent cyanation protocol, the synthesis of 2 a was carried out on a 5.0 mmol scale, resulting in comparable yield and enantioselectivity to the 0.5 mmol scale reactions.
Having implemented the new strategy to employ alkylboronic esters in enantioconvergent cyanation, we next sought to delineate the reaction mechanism and lay the groundwork for our future expansion of the oxidative cross-coupling platform. Specifically, through a combined experimental and computational approach, we are interested in (1) probing our hypothesized aniline-assisted C−B bond homolysis for alkyl radical generation, (2) examining the electronic structure features of the reactive organometallic intermediate that enables the cross-coupling reactivity, and (3) elucidating potential pathway20 for transition metal-mediated radical functionalization.21
Mechanistic Studies of Aniline-Assisted Alkyl Radical Generation
Cyanation of radical clock substrate 4 led to the formation of both direct cyanation product 5 a as well as ring-opening product 5 b (Figure 2A), suggesting the intermediacy of an alkyl radical. Subjecting enantiomerically enriched (R)-1 a to the cyanation conditions with either (+)-L1 or (−)-L1 as the ligand resulted in the formation of product 2 a with similar yields and opposite configurations (Figure 2B), indicating the loss of stereochemical information following alkyl radical formation.
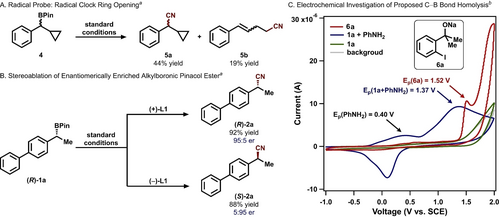
Mechanistic probes for the enantioconvergent deborylative cyanation reaction: (A) The proposed alkyl radical formation is supported by radical ring opening of the cyclopropyl substituent in substrate 4; (B) Stereochemical information is lost after the generation of an alkyl radical; and (C) Electrochemical studies support the thermodynamic feasibility of the proposed single-electron alkylboronic pinacol ester activation; inset: sodium 2-(2-iodophenyl)propan-2-olate (6 a). aReaction carried out on a 0.20 mmol scale. Reaction yields were determined by 1H NMR spectroscopy of the crude reaction mixture using 1,1,2,2-tetrachloroethane as an internal standard. bCyclic voltammograms are obtained in 1,2-difluorobenzene at 25 °C with 0.1 M (nBu4N)(PF6); 100 mV s−1; referenced to SCE.
Electrochemical studies were carried out to examine the mechanism of aniline-assisted C−B bond homolysis. 1,2-Difluorobenzene was used as the solvent for its ability to effect the cyanation, albeit with a lower yield (Table 1, entry 8).22 To evaluate the thermodynamic driving force for the single-electron activation of 1 a, we measured the cyclic voltammogram of alkoxide 6 a and observed an irreversible oxidation event at 1.52 V (vs. SCE, Figure 2C, red trace). No oxidation event of 1 a was observed within the solvent potential window (green trace), consistent with the high oxidation potential of alkylboronic pinacol esters13a and the necessity of a Lewis basic additive (Table 1, entry 15). Upon the addition of a stoichiometric amount of aniline (Ep=+0.40 V vs SCE),23 an irreversible oxidation event was observed with a peak potential of +1.37 V (vs SCE, blue trace).14b The new oxidation event could be attributed to (1) the formation of an aniline-1 a adduct14 or (2) the association of 1 a with in situ generated aminyl radical (Figure 3, vide infra)24 and the subsequent cleavage of C−B bond.
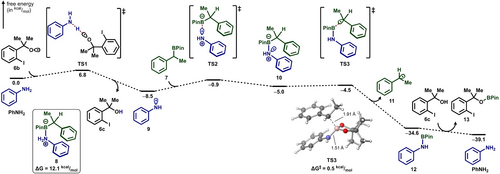
B3LYP-D3(BJ)/6-311+G(d,p)/SMD (benzene) computed Gibbs energy profile of aniline-assisted generation of benzylic radical 11.
The feasibility of these two pathways for alkyl radical generation was assessed through computational studies using 1-phenylethyl boronic pinacol ester (7). We first considered the formation of an aniline-7 adduct (8). Computational analysis indicated that the binding of aniline to 7 is endergonic (ΔG=12.1 kcal/mol) with an activation barrier of 14.0 kcal/mol (Scheme S5). However, single electron transfer between 8 and alkoxy radical 6 b proceeding C−B bond homolysis is highly thermodynamically unfavorable (ΔG=52.6 kcal/mol, Scheme S6). Alternatively, the formation of an aminyl radical 9 through hydrogen atom transfer (HAT) to 6 b was computed to be exergonic (ΔG=−8.5 kcal/mol) with a small kinetic barrier (ΔG≠=6.8 kcal/mol, Figure 3). Association of the resulting aminyl radical 9 with 7 leads to adduct 10 (ΔG=+3.5 kcal/mol) from which homolytic substitution (SH2) can occur easily. We found a flat potential energy surface (PES) in which this occurs in a formal stepwise fashion, albeit with a shallow intermediate that is tetravalent at the boron center (10). Subsequent facile C−B bond scission (ΔG≠=0.5 kcal/mol) from 10 produces alkyl radical 11 in a highly exergonic fashion (ΔG=−29.6 kcal/mol). The computed low barriers of this pathway are also consistent with the reported alkyl radical generation from reactions between alkylboronic pinacol esters and aminyl radicals.13c
Spectroscopic Characterization and Electronic Structure Analysis of Reactive Organometallic Intermediates
Upon formation of the prochiral alkyl radical, we hypothesized that a ligated cupric dicyanide complex (L1)Cu(CN)2 (14) enables the enantioselective cyano group transfer.15a, 25 We propose that the highly covalent nature of Cu(II)–CN bond distributes the open-shell character across both the metal center and cyano ligand, enabling its single-electron cyano group transfer reactivity. Intermediate 14, however, has yet to be spectroscopically characterized. In order to access 14, complex (L1)CuOAc (P1) and (L1)Cu(OAc)2 (15, Figure S8) were prepared and structurally characterized. Exposing P1 to stoichiometric TMSCN in benzene resulted in a white suspension over two minutes (Figure S3). We propose that the white precipitate is (L1)Cu(CN) (16) in the form of an insoluble coordination polymer.26 Infrared spectroscopy measurement of the solid product revealed a C≡N stretch of 2102 cm−1, consistent with previously characterized four-coordinate Cu(CN) coordination polymers.27 The sequential addition of 3 and TMSCN to 16 or treatment of 15 with two equivalents of TMSCN instantaneously resulted in a dark purple solution, potentially due to the formation of 14. A similar observation of the formation of a purple species was reported in the synthesis of complex (bpy)Cu(CN)2.28 The paramagnetic species 14 is highly unstable even at −78 °C (in toluene or 2-methyltetrahydrofuran), with the purple solution effervescing and changing into a white suspension within seconds.29 IR measurement of the resulting white precipitate confirmed its identity as 16. This observation is consistent with the redox buffering ability of the cyanide nucleophile in facilitating radical relay reactions.30 The thermal instability of 14 limited our attempts at its isolation and structural characterization. Instead, 14 was characterized using EPR spectroscopy under cryogenic conditions, and its electronic structure was probed using DFT calculations. The X-band CW-EPR spectrum of 14 collected at 77 K in toluene revealed an S=1/2 spin state with the unpaired electron occupying a pseudo-axial environment (Figure S4, g1=2.306, g2=2.083). A hyperfine constant of A1,Cu=379.3 Hz was derived from the g1=2.306 signal. EPR spectroscopic analysis of a catalytic reaction mixture after 30 min revealed a similar signal. Computational DFT studies corroborated these experimental data, indicating that the formation of 14 from either 15 in the presence of two equivalents of TMSCN or the sequential addition of 3 and TMSCN to 16 is thermodynamically favorable (ΔG=−12.8, −20.4 kcal/mol, respectively. Schemes S13 and S14).
In an open-shell system, ligand-based spin density contributes to a range of radical-type reactivities, such as the H-atom abstraction and radical rebound in C−H hydroxylation catalyzed by cytochrome P450.31 We hypothesized that the facile radical cyano group transfer reactivity of 7 similarly results from a significant degree of ligand-based radical density. From a molecular orbital perspective, the unpaired electron in 14 resides in a singly occupied molecular orbital (SOMO) with contributions from both the metal 3d and carbon 2p orbitals. To gain insight into the electronic structure and frontier molecular orbitals of the Cu(II) biscyano complex 14, particularly the distribution of the unpaired electron across the Cu−CN σ-bond, natural bonding orbital (NBO)32-based natural population and intrinsic bonding orbital (IBO)33 analyses were performed. Natural population analysis of 14 indicated that the unpaired electron in the SOMO is delocalized across the central Cu atom (0.513) and the carbons of the two cyano ligands (0.365, see Scheme S15). Effective oxidation state (EOS)34 calculations for 14, performed at the B3LYP(D3-BJ)/def2-TZVPPD level of theory, closely corroborated these data showing a partial spin delocalization on Cu (0.569) and the cyano carbons (0.310) (Figure S20). NBO-based orbital composition analysis of the highest occupied alpha SOMO of 14 features a significant contribution from cyanide carbon atoms (44.0 %) compared to the copper center (14.9 %) (Figure 4), a result from the highly covalent nature of the Cu−C bond.
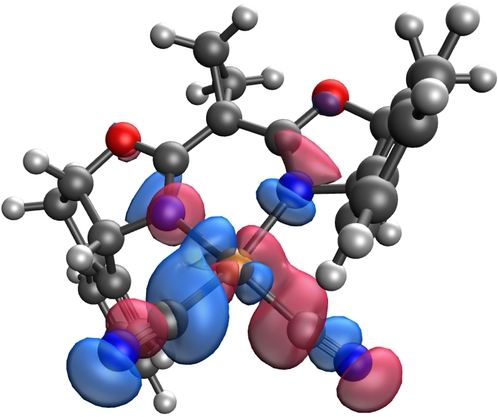
SOMO of 14; see Supporting Information for details.
Theoretical Calculation of the Cu-Catalyzed Cyanation Pathway
Computational studies were next carried out to better understand the proposed mechanism of this transformation and to elucidate the origins of enantioconvergence. Dispersion-corrected DFT calculations were performed at the B3LYP-D3(BJ) level of theory with 6-311+G(d,p)//LANL2DZ (F, I)//LANL2TZ (Cu) basis sets using an implicit SMD model of benzene.35 We modeled the cyanation of 1-phenylethyl boronic pinacol ester (7) with ligand (+)-L1. DFT calculations, in conjunction with experimental studies, indicated that the formation of catalytically active 14 was expected to proceed exergonically from complex 16 (ΔG=−25.9 kcal/mol, Figure 5). A stepwise pathway was computed. The oxidation of 16 by fluorine atom transfer from hypervalent iodine reagent 3 furnishes complex 17 and oxygen-based radical 6 b (ΔG=−10.8 kcal/mol).36 Subsequent ligand exchange between 17 and TMSCN forms 14 in an exergonic fashion (ΔG=−15.1 kcal/mol) through a four-membered ligand exchange transition state TS4.
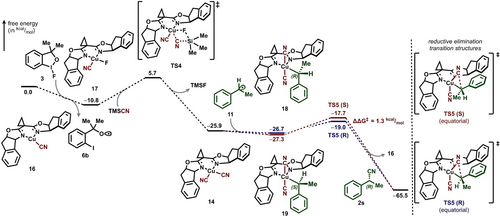
B3LYP-D3(BJ)/6-311+G(d,p)//LANL2DZ (F, I)//LANL2TZ (Cu) computed Gibbs energy profile of enantioconvergent deborylative cyanation via an inner-sphere mechanism via a formal Cu(III) intermediate (kcal/mol).
DFT calculations indicated that the formation of a formal Cu(III) intermediate can occur in one of two binding modes, with 11 assuming either a pseudoequatorial or -apical position with respect to the BOX ligand (Figure 5). Conformational analysis of both binding modes revealed that placing the benzylic group in the less-congested equatorial position and the electronegative cyano groups in the apical positions as in 18 and 19 is more favorable (Scheme S3). In contrast, addition of 11 along the apical direction of 14 results in more thermodynamically disfavored intermediates (ΔΔGpro-R=6.9 kcal/mol, ΔΔGpro-S=9.1 kcal/mol, Scheme S4). This is likely the result of (1) a more sterically congested coordination environment and (2) the strong trans effect between the cyano ligands and alkyl groups. Transition states corresponding to the formation of 18 and 19 cannot be located despite attempts at various levels of theory and broken symmetry calculations.37 The relaxed potential energy surface scan by modulating the Cu−C distance showed no inflection point, consistent with previous studies (see Supporting Information for details).37b
The subsequent C−C bond formation was calculated to occur via reductive elimination transition structures (TSs) TS5 (S) and TS5 (R) with retention of configuration at the benzylic carbon. Assuming reversible formation of the diastereomeric formal Cu(III) complexes 18 and 19, the reductive elimination is enantiodetermining. Application of the Curtin Hammett principle results in a ΔΔG≠ of 1.3 kcal/mol in favor of the major R-enantiomer, consistent with our experimental observation.15a, 37b We ascribe the preferential formation of the R-enantiomer to additional stabilizing dispersion interactions in the reductive elimination TSs (Figure S23). Direct benzylic radical attack on the cyano groups in 14 following an outer-sphere pathway was also probed computationally. The result reveals a diminished ΔΔG≠ of 0.6 kcal/mol favoring the R-enantiomer and suggests that the inner-sphere pathway is kinetically more favorable (see Supporting Information for details).
Our combined experimental and computational mechanistic studies led to the following proposed catalytic cycle (Figure 6). Single electron oxidation of Cu(I) catalyst 16 generates concurrently alkoxy radical 6 b and Cu(II) fluoride complex 17. Hydrogen atom transfer between 6 b and aniline produces aminyl radical 9, while transmetalation between 17 and TMSCN furnishes reactive Cu(II)(CN)2 intermediate 14. The association between aminyl radical 9 and substrate 7 followed by C−B bond homolysis leads to the prochiral alkyl radical 11, which subsequently reacts with Cu(II)(CN)2 intermediate 14 to generate enantiomerically enriched product 2 s.
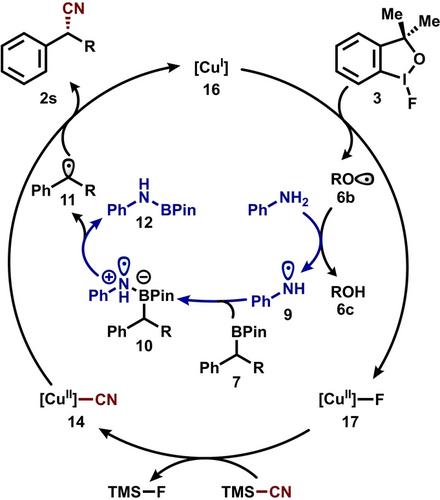
Proposed catalytic cycle.
Conclusions
In summary, we have developed a Cu-catalyzed enantioconvergent deborylative cyanation protocol. This method is tolerant of a wide range of functional groups and heterocycles. The cyanation product can be obtained on a gram scale with a high level of enantiomeric enrichment. Mechanistic evidence suggests the generation of a prochiral radical in a stereoablative fashion, followed by enantioselective radical trapping by a Cu(II)(CN)2 intermediate. Electronic structure analysis of an in situ generated Cu(II)(CN)2 reactive intermediate suggests an evenly distributed spin density across the Cu center and cyano ligands. DFT calculation corroborates our proposed radical-relay mechanism and suggests reductive elimination as the enantiodetermining step.
Acknowledgments
This work was supported by startup funds from Colorado State University. R.S.P. acknowledges support from the NIH (R01 GM151533) and computational resources from the Alpine high-performance computing resource jointly funded by the University of Colorado Boulder, the University of Colorado Anschutz, and Colorado State University, and ACCESS through allocation TG-CHE180056. We are grateful to Drs. Jeffery Bandar, Sheng Feng, and Elaine Raguram for advice on the preparation of this manuscript.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data supporting the findings of this study are available in the Supporting Information. Details about materials and methods, experimental procedures, characterization data, NMR spectra, computational methods, additional computational data, DFT energies, and Cartesian coordinates for all optimized structures are available in the Supporting Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2314505 (for P1), 2314506 (for 15), 2314507 (for 2 b), 2314508 (for 2 u). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.