α’-Selective Selenium-catalyzed Allylic C−H Amination of Enol Derivatives
Graphical Abstract
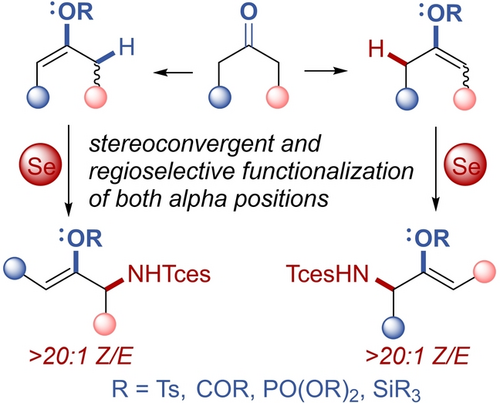
A selenium catalyst allows C−H amination of a wide range of potentially cross-reactive enol derivatives with unique selectivity for the α’-position, thereby unlocking the reactive potential of both ketone α-positions. Stereoconvergent formation of Z enol derivatives from E/Z mixtures eliminates the need for selective enolization methods. Resonance donation from the oxygen in the ene transition state is responsible for the high regioselectivity.
Abstract
A transition metal-free Se-catalyzed C−H amination protocol for α’-amination of enol derivatives has been developed. This reaction can be used to functionalize a wide variety of oxygen- and halogen-substituted alkenes spanning a vast range of nucleophilicities, giving α’-aminated enol derivatives with high regioselectivity. Amination of E/Z mixtures of alkenes proceeds stereoconvergently to give the (Z)-enol derivatives exclusively. Mechanistic studies revealed that the relative reactivity and α’-regioselectivity of these transformations is determined by substantial resonance donation to the heteroatom-bound carbon in the transition state. These products participate in traditional reactions of enol derivatives, allowing for efficient functionalization of both α- and α’-positions from a single enol derivative with high diastereocontrol.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.