Organic Donor–Acceptor Thermally Activated Delayed Fluorescence Photocatalysts in the Photoinduced Dehalogenation of Aryl Halides
Graphical Abstract
A family of donor-acceptor thermally activated delayed fluorescence compounds based on DMAC-TRZ and containing aryl subtituents on the DMAC donor have been applied as photocatalysts in the dehalogenation of aryl halides having Ered up to −2.72 V vs SCE. Mechanistic studies indicate that these DMAC-TRZ based compounds act as pre-photocatalysts and photodegrade under the reaction conditions, with the photodecomposition product responsible for the photocatalysis.
Abstract
We report a family of donor-acceptor thermally activated delayed fluorescent (TADF) compounds based on derivatives of DMAC-TRZ, that are strongly photoreducing. Both Eox and thus E*ox could be tuned via substitution of the DMAC donor with a Hammett series of p-substituted phenyl moieties while Ered remained effectively constant. These compounds were assessed in the photoinduced dehalogenation of aryl halides, and analogues bearing electron withdrawing groups were found to produce the highest yields. Substrates of up to Ered=−2.72 V could be dehalogenated at low PC loading (1 mol %) and under air, conditions much milder than previously reported for this reaction. Spectroscopic and chemical studies demonstrate that all PCs, including literature reference PCs, photodegrade, and that it is these photodegradation products that are responsible for the reactivity.
Introduction
Photocatalysis has evolved over the last 15 years to be a popular and effective methodology of organic synthesis, enabling the formation of compounds that would otherwise be inaccessible using traditional thermal synthesis.1-3 Moreover, photocatalysis is arguably a “greener” route of synthesis as conditions are generally less harsh (ambient temperature and pressure) and often avoids the use of toxic stoichiometric oxidants and/or reductants, in favour of catalytic amounts of the electron transfer agent, the photocatalyst (PC).
Particularly, the mild generation of aryl radicals using photocatalysis has received significant interest,4 especially since classic methods of forming these species from aryl halides involve stoichiometric reagents such as AIBN/n-Bu3SnH.5, 6 Instead, in photocatalysis, low loadings of the PC are used to directly photoreduce the aryl halide, releasing a reactive aryl radical. However, this requires a strong photoreductant for scission of the C−X bond. The organometallic PC, fac-Ir(ppy)3 (excited-state oxidation potential, E*ox−1.70 V vs SCE), was shown to undergo hydrodeiodination of aryl iodides,7 however the toxicity and scarcity of the transition metal has prompted considerable efforts to identify organic alternatives as PCs that do not suffer these same issues. A small series of organic PCs have thus been reported to initiate photoinduced dehalogenation of aryl halides over the last 10 years,8-16 a selection of which are shown in Figure 1a. Both N,N-bis(2,6-diisopropylphenyl)perylene-3,4,9,10-bis(dicarboximide) (PDI)8 and 10-phenylphenothiazine (PTH)9 (Figure 1a) could dehalogenate aryl iodides with both electron-withdrawing groups (EWGs) Ered≈−1.0–−1.7 V vs SCE11, 17, 18 and electron-donating groups (EDGs) Ered > −2.3 V vs SCE,19 but could only dehalogenate aryl bromides and chlorides with EWGs present Ered≈−1.80–−2.0 V vs SCE;20 exceptionally, PTH could dehalogenate 2-bromophenol in a low yield (23 % over 72 h). Both PDI and PTH were used at 5 mol % loading, under a N2 atmosphere (Figure 1b), although PTH was shown to be somewhat tolerant to air (94 to 68 % yield under N2 and air, respectively, in the dehalogenation 4-chlorobenzonitrile).
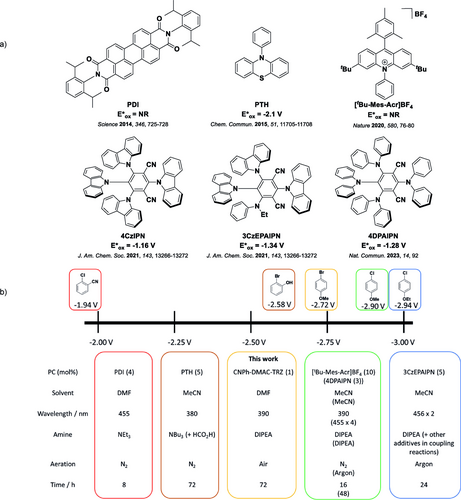
a) A selection of organic PCs used in the literature for photoinduced dehalogenation of aryl halides and b) a summary of the most difficult substrates that can be reduced using these PCs and for the PC used in this work, as well as the conditions for that specific substrate. References given in a) are related to the relevant dehalogenation literature and are the corresponding references for the conditions shown in b). NR=not reported. Redox potentials quoted vs SCE and taken from Ref. [17, 19–21, 23, 24].
Recently, significant progress in the dehalogenation of aryl bromides and chlorides containing EDGs (Ered<−2.50 V vs SCE)21 has been achieved. Notably, Nicewicz and co-workers used an acridinium salt 3,6-di-tert-butyl-9-mesityl-10-phenylacridin-10-ium tetrafluoroborate, hereby named [tBu−Mes−Acr]BF4 (Figure 1a)10 to achieve yields of 58–99 % over a wide scope of aryl bromides and chlorides containing EDGs; however, a high 10 mol % PC loading was required (Figure 1b). Cyanoarene based PCs, including 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene, 4CzIPN, and related derivatives (Figure 1a),11, 12 have been used in dehalogenation reactions involving aryl bromides and chlorides with EDGs, in yields of 38–74 % for direct dehalogenation11 and 20–90 % in coupling reactions.12 Significant photon flux in the form of two or four LEDs (2 40 W12 or 4 3 W11), and a PC loading of 5 mol % were used to attain these yields (Figure 1b).22
The proposed mechanisms for such photoinduced dehalogenation involve either an oxidative quenching cycle or a consecutive photoinduced electron transfer (conPET) process (Figure 2). In the former, direct photoreduction of the aryl halide occurs from the PC*, with closure of the photocatalytic cycle proceeding through oxidation of the sacrificial amine by PC⋅+. In contrast, the conPET mechanism involves reductive quenching of the PC* by the sacrificial amine, generating PC⋅−, which itself is then photoexcited. The [PC⋅−]* then reduces the aryl halide, regenerating the ground state PC.
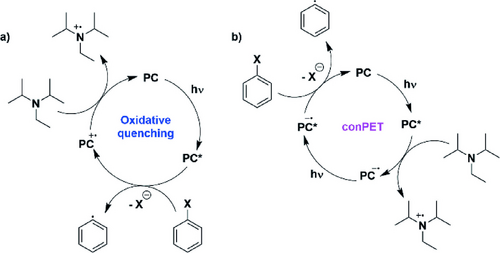
Of the organic PCs discussed, only PTH has been proposed to dehalogenate aryl halides through an oxidative quenching cycle, this based on Stern–Volmer quenching studies where only iodobenzene, not NBu3, could quench the emission of PTH.9 However, despite E*ox=−2.10 V vs SCE for PTH, it was shown to dehalogenate 2-bromophenyl (Ered=−2.58 V vs SCE),21 a transformation which would be considerably endergonic. PDI, [tBu−Mes−Acr]BF4 and 3CzEPAIPN (and related derivatives) have all been suggested to operate via the conPET mechanism. The evidence to support this stems from the quadratic dependency of yield with light irradiation intensity, and the direct detection of PC⋅−, by EPR spectroscopy, as an intermediate in the photocatlysis.12
Despite the advances that have been made in the photoinduced dehalogenation of aryl halides, the reaction conditions employed often use high organic PC loading (5–10 mol %) and multiple light sources. As part of a larger research effort devoted to the design of highly photooxidizing and photoreducing PCs, we sought to demonstrate the utility of a new family of donor-acceptor thermally activated delayed fluorescence (TADF) compounds as PCs that would be capable of promoting this reaction under milder conditions.
TADF compounds have received increasing interest for their use as PCs,25-28 particularly 4CzIPN.29 TADF compounds are typically composed of donor and acceptor units that are electronically weakly coupled, usually due to a strongly twisted conformation.30 Thus, modifications of the donor and acceptor motifs adjust the HOMO and LUMO levels, respectively, facilitating a facile tuning of the optoelectronic properties of the compounds. With this in mind, we focused on 10-(4-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl)-9,9-dimethyl-9,10-dihydroacridine, DMAC-TRZ (Figure 3a). First developed in 2015 as a sky-blue emitter for organic light-emitting devices,31 DMAC-TRZ is composed of a DMAC donor connected to a triazine acceptor. We identified DMAC-TRZ as a potentially useful photoreductant as it has a moderate optical gap (E0,0=2.50 eV in DMF), and ground-state oxidation potential (Eox=0.97 V vs SCE in DMF), leading to an E*ox of −1.53 V (c.f. E*ox=−1.7 V vs SCE for fac-Ir(ppy)3).9 Additionally, it is commercially available at a significantly lower price than 4CzIPN (£147.00 for 200 mg compared to £864.00 per 250 mg for 4CzIPN).32 Due to the near orthogonal conformation adopted by the donor, the oscillator strength for the lowest energy charge-transfer (CT) transition is exceedingly low, which translates into a low-intensity low-energy absorption band (λabs=381 nm, ϵ=2000 M−1 cm−1 in DMF).
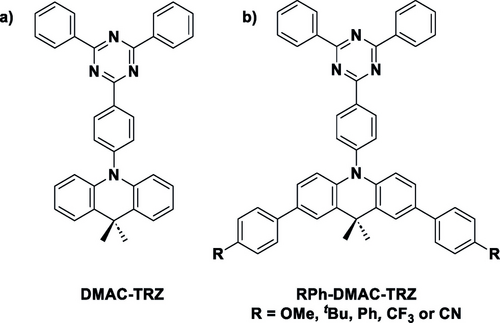
Structures of a) DMAC-TRZ and b) functionalized DMAC-TRZ derivatives.34
To rationally and progressively tune the optoelectronic properties of this compound, we substituted the DMAC donor at the 2- and 7-positions with a Hammett series of 4-substituted phenyl moieties (Figure 3b),33 which should affect Eox, E0,0, and thus E*ox.34
Results and Discussion
Optoelectronic Characterisation
The optoelectronic properties of the DMAC-TRZ derivatives were ascertained in DMF, a solvent that is frequently used in literature dehalogenation reactions; the poor solubility of these DMAC-TRZ derivatives in MeCN precluded its use. The UV/Vis absorption spectrum of DMAC-TRZ (Figure 4a) matches that previously recorded in toluene,31 displaying a weak band between 350–450 nm, assigned to the intramolecular CT transition from DMAC to TRZ. Excluding CNPh-DMAC-TRZ, the other four derivatives display a weak low-energy CT absorption band that maintains a similarly low ϵ to DMAC-TRZ (Figure 4a); however, when changing the DMAC substituent from EWG to EDG, the onset of the CT band progressively red-shifts. All five derivatives have a more red-shifted onset relative to DMAC-TRZ indicating the increased conjugation within the donor.
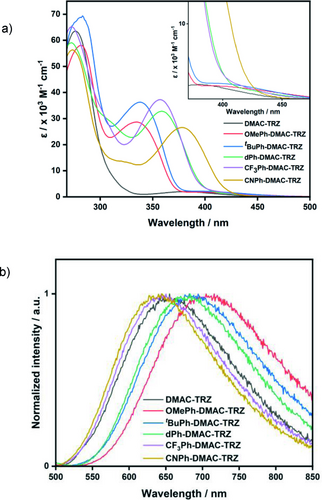
a) UV/Vis absorption and b) steady-state PL of DMAC-TRZ and derivatives obtained in DMF under air. The inset in a) shows a zoomed in section of the CT absorption band.
In addition to this CT band, the absorption spectra of the five DMAC-TRZ derivatives have a well-defined band between 335–378 nm, which is assigned to a locally-excited (1LE) transition on the substituted DMAC donor.34 Unlike the trend for the CT band, the LE band becomes more red-shifted when moving from EDG to EWG on the DMAC donor (e.g., λabs=335 and 378 nm for OMePh-DMAC-TRZ and CNPh-DMAC-TRZ, respectively). The LE band is approximately ten-fold more intense than the CT peak (36 103 and 2.1 103 M−1 cm−1 for the LE and CT peaks, respectively, for tBuPh-DMAC-TRZ) allowing for the substituted DMAC-TRZ derivatives to be more absorptive at 390 nm than the parent DMAC-TRZ.
Steady-state photoluminescence (PL) spectra in DMF for the five DMAC-TRZ derivatives (Figure 4b and Table 1) are broad and Gaussian-shaped, indicative of emission from a CT state. The emission progressively red-shifts across the family in a similar manner to the CT band in the absorption spectra (e.g., λPL=645 nm and 716 nm for CNPh-DMAC-TRZ and OMePh-DMAC-TRZ, respectively).
Compound |
λPL/nm |
E0,0/eV |
Eox/V [b] |
Ered/V |
E*ox/V [c] |
E*red/V |
τp/ns |
τd/ns |
|---|---|---|---|---|---|---|---|---|
DMAC-TRZ |
655 |
2.50 |
0.97 |
−1.59 |
−1.53 |
0.91 |
3.2 (2.3) |
954 (52) |
OMePh-DMAC-TRZ |
716 |
2.29 |
0.79 |
−1.58 |
−1.50 |
0.71 |
|
|
tBuPh-DMAC-TRZ |
682 |
2.35 |
0.81 |
−1.58 |
−1.54 |
0.77 |
|
|
dPh-DMAC-TRZ |
683 |
2.39 |
0.86 |
−1.58 |
−1.53 |
0.81 |
|
|
CF3Ph-DMAC-TRZ |
644 |
2.47 |
1.01 |
−1.57 |
−1.46 |
0.90 |
4.1 (3.8) |
260 (65) |
CNPh-DMAC-TRZ |
645 |
2.70 |
1.04 |
−1.58 |
−1.66 |
1.12 |
5.2 (4.8) |
810 (62) |
- [a] Values are reported in DMF unless otherwise noted. E0,0 determined from the intersection point from the tangents of the onsets of the absorption and emission spectra. Redox potentials are reported vs SCE and are obtained from the maxima of the oxidation and reduction waves from the DPV. Eox*=Eox−E0,0 and Ered*=Ered+E0,0. τp and τd refer to prompt and delayed lifetimes, respectively. Lifetimes are obtained under N2, with the value in parentheses being obtained under air. [b] Obtained in DCM. [c] Calculated using the Eox value in DCM and the E0,0 value in DMF.
The optical gap, E0,0(S1) was determined from the intersection point of the tangents from the onsets of the absorption and emission spectra (Table 1, Figure S3–S8). Generally, the E0,0 energy increases from EDG to EWG, for example, E0,0=2.29 and 2.70 eV for OMePh-DMAC-TRZ and CNPh-DMAC-TRZ, respectively, as demonstrated in Figure 5.
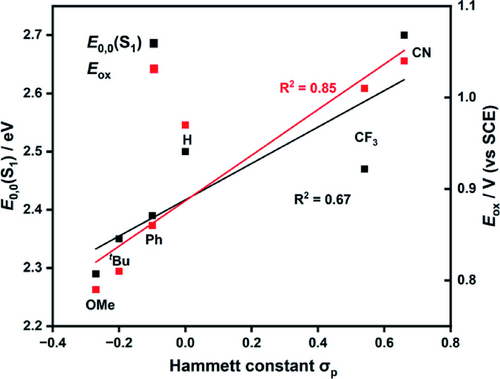
Correlation of optical gap E0,0(S1) and Eox of the DMAC-TRZ derivatives with Hammett constant. The black R2 corresponds to the black trendline associated with E0,0(S1) and the red R2 to the red trendline associated with Eox.
Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) revealed that DMAC-TRZ and its derivatives have similar reduction potentials, Ered, at ca. −1.58 V vs SCE in DMF (Figure S9), reflecting that reduction occurs on the triazine acceptor moiety and the donor groups are effectively electronically decoupled in the ground state. The Ered value in DMF is shifted anodically by ca. 100 mV relative to the Ered value previously reported in DCM (Ered=−1.69 V vs SCE in DCM).34 The limited electrochemical solvent window of DMF in the oxidation region prevented direct observation of the oxidation potential, Eox. Rather, the Eox values in DCM were used as a surrogate (Figure S4, Table 1).34 When changing from EDG to EWG substituted DMAC-TRZ, Eox shifted anodically (e.g., OMePh-DMAC-TRZ, Eox=0.79 V and CNPh-DMAC-TRZ, Eox=1.04 V), as shown in Figure 5. From the electrochemical data, it can be concluded that all six DMAC-TRZ-based compounds are equally reducing in the PC⋅− state, but PC⋅+ will become progressively more oxidizing as the substituent changes from EDG to EWG on the DMAC.
Despite having effectively the same Ered values, the six compounds display a range of E*red values (Table 1) due to the varying E0,0. The DMAC-TRZ derivatives become more potent photooxidants when changing from EDG (OMePh-DMAC-TRZ, E*red=0.71 V) to EWG (CNPh-DMAC-TRZ, E*red=1.12 V); however, show similar E*ox values (−1.46 V to −1.54 V) due to compensatory changes in Eox and E0,0. The outlier compound, CNPh-DMAC-TRZ, is significantly more photoreducing (E*ox=−1.66 V), comparable to fac-Ir(ppy)3 (E*ox=−1.70 V), although is less photoreducing than PTH (E*ox=−2.10 V).9
The time-resolved PL decays were obtained for a subset of the DMAC-TRZ derivatives in DMF under N2 and air (Figures S11–14). As is characteristic of TADF compounds, the emission decays with biexponential kinetics associated with direct radiative decay from the S1 state and delayed emission originating from the same state following intersystem crossing/reverse intersystem crossing cycles. Under deaerated conditions, the prompt lifetimes, τp, of DMAC-TRZ, CF3Ph-DMAC-TRZ and CNPh-DMAC-TRZ, are in the nanosecond region (3.2–5.2 ns, Table 1), while the delayed lifetimes, τd, are in the microsecond regime (0.2–0.9 μs). These compounds retain the delayed emission even under air, although τd decreases dramatically to 52–65 ns.
Photochemical Dehalogenation of Aryl Halides
The photoreducing ability of these compounds was probed in the dehalogenation of aryl halides, initially using methyl 4-chlorobenzoate (Ered=−1.98 V in DMF vs SCE) as a model substrate. Reaction yields were determined by GC-MS; the PCs were not detectable using this method. To enable a comparison to the literature yields, the conditions of Discekici et al. were first closely replicated using PTH (5 mol %) as the PC, MeCN as solvent, and NBu3 and HCO2H as additives. The literature yield using PTH (94 %) was higher than that achieved by us (77 % Table 2, entry 1), likely owing to the different reaction times (72 h vs 18 h, respectively). However, since most literature reports use only an amine as the additive (Figure 1b), which is often DIPEA, the reaction conditions were modified to omit the acid and replace NBu3 with DIPEA, resulting in a 42 % yield with PTH (Table 2, entry 2). As the DMAC-TRZ derivatives are not soluble in MeCN, the solvent was changed to DMF, giving a 78 % yield (Table 2, entry 3). Finally, at a reduced PC loading of 1 mol % of PTH, the yield decreased to 61 % (Table 2, entry 4).
|
||
Entry |
PC |
GC-MS yield/% |
|---|---|---|
1 |
PTH |
77 1[b] |
2 |
PTH |
42 4[c] |
3 |
PTH |
78 4[d] |
4 |
PTH |
61 4 (17 1) |
5 |
DMAC-TRZ |
43 2 (36 0) |
6 |
OMePh-DMAC-TRZ |
55 3 (2 2) |
7 |
tBuPh-DMAC-TRZ |
64 0 (79 2) |
8 |
dPh-DMAC-TRZ |
100 4 (100 2) |
9 |
CF3Ph-DMAC-TRZ |
98 2 (94 2) |
10 |
CNPh-DMAC-TRZ |
100 0 (98 2) |
11 |
OMePh-DMAC-TRZ |
70 0 [e] |
12 |
None |
0 0 |
13 |
DMAC-TRZ |
0 0 [f] |
14 |
DMAC-TRZ |
0 0 [g] |
- [a] Reaction conditions: aryl halide (1 equiv., 0.1 mmol), PC (1 mol %) DIPEA (5 equiv.) and DMF (1 mL) at room temperature under N2 for 18 h with irradiation from 390 nm Kessil LED. GC-MS yields were determined using mesitylene as an internal standard. Values in parentheses were conducted under aerated conditions. Yields represent the average yield obtained from at least two independent runs, with the associated standard deviation. [b] 5 mol % of PC, NBu3 (5 equiv.) and HCO2H (5 equiv.) and MeCN were used under N2. [c] 5 mol % of PC, DIPEA (5 equiv.) and MeCN were used under N2. [d] 5 mol % of PC, DIPEA (5 equiv.) and DMF were used under N2. [e] 48 h reaction time. [f] No light. [g] No amine.
The DMAC-TRZ derivatives were then trialled in the reaction using these conditions (DIPEA as the additive, DMF the solvent and 1 mol % of PC). Pleasingly, all compounds dehalogenated the substrate (Table 1, entries 5–10), and the substituted DMAC-TRZ derivatives afforded higher product yields than DMAC-TRZ (55–100 % and 43 %, respectively). The GC-MS yields were highest with derivatives possessing EWGs, which may be a consequence of these being more absorptive at the excitation wavelength (390 nm, a); this may influence the reaction kinetics. Thus, the dehalogenation of the substrate using OMePh-DMAC-TRZ as the PC was conducted for 48 h, resulting in an improvement in yield from 55 % (18 h irradiation) to 70 % (Table 2, entry 11). Control reactions indicated that no product is formed in the absence of PC, amine, or light (Table 2, entries 12–14).
To increase the dehalogenation yield with DMAC-TRZ, other amines, PC loadings and solvents were screened (Table S2 and S3); however, no improvement in product yield was observed. Additionally, the tolerance of the reaction to air was investigated; all the PCs, except OMePh-DMAC-TRZ, performed equally well under aerated conditions (Table 2, entries 5–10). Therefore, subsequent reaction scope was conducted using DIPEA as the amine, DMF as the solvent, and aerated conditions.
All DMAC-TRZ derivatives were assessed in the substrate scope, except for OMePh-DMAC-TRZ, which was not tolerant to air under the reaction conditions, and the performance was cross compared with 4CzIPN and PTH. The dehalogenation of aryl bromides and chlorides bearing EWGs, (Ered=−1.88 to -−2.04 V vs SCE, Figure 6a–d), was first considered. Methyl 4-chlorobenzoate and 4-chlorobenzonitrile have previously been dehalogenated by PTH at 5 mol % loading, 380 nm irradiation over 72 h, with NBu3 and HCO2H as the additives in MeCN, to give 94 and 94 % yield, respectively.9 As discussed above, replicating these conditions but with a shorter reaction time of 18 h, produced a 77 % yield of methyl benzoate from methyl 4-chlorobenzoate. Instead, under the optimized conditions identified for the DMAC-TRZ derivatives, the use of PTH yielded only 17 and 13 % of the dehalogenated product, respectively, from methyl 4-chlorobenzoate and 4-chlorobenzonitrile. The DMAC-TRZ derivatives yielded high quantities of the dehalogenated products under these conditions, particularly CNPh-DMAC-TRZ, which quantitatively dehalogenated these two substrates. In fact, the use of CNPh-DMAC-TRZ yielded 88–100 % of the target product for all four substrates with Ered of −1.88 V to −2.04 V (Figure 6). CF3Ph-DMAC-TRZ and dPh-DMAC-TRZ also performed moderate to excellently for these substrates (50–94 % and 45–100 % yield, respectively), tBuPh-DMAC-TRZ tended to give lower yields (35–79 %) and DMAC-TRZ was the least efficient (8–36 %). Importantly, across all substrates, at least one of the DMAC-TRZ derivatives afforded a greater yield than 4CzIPN and PTH.
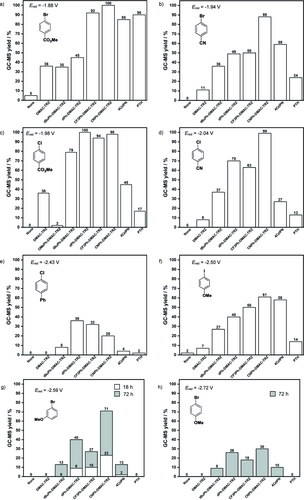
GC-MS yields of dehalogenated products using either no PC or one of DMAC-TRZ, tBuPh-DMAC-TRZ, dPh-DMAC-TRZ, CF3Ph-DMAC-TRZ, CNPh-DMAC-TRZ, 4CzIPN and PTH. Reaction conditions unless otherwise stated: aryl halide (1 equiv., 0.1 mmol), PC (1 mol %) DIPEA (5 equiv.) and DMF (1 mL) at room temperature under air for 18 h with irradiation from 390 nm Kessil LED. GC-MS yields were determined using mesitylene or 1,3,5-trimethoxybenzene as the internal standard. All redox potentials are quoted vs SCE and are from Ref. [17, 19–21, 23] a) Reaction was conducted under N2. g) Reaction was conducted for 18 h and 72 h. h) Reaction conducted for 72 h.
We next attempted the dehalogenation of more demanding aryl halides bearing conjugative or EDGs. The dehalogenation of 4-chlorobiphenyl (Ered=−2.43 V, Figure 6e) was screened; dPh-DMAC-TRZ, CF3Ph-DMAC-TRZ and CNPh-DMAC-TRZ afforded low yields of product (36, 32 and 20 %, respectively). tBuPh-DMAC-TRZ performed even worse (8 %) while DMAC-TRZ did not produce any reaction. The use of 4CzIPN or PTH resulted in <5 % of product. The low yields obtained are tentatively attributed to alternative side reactions that seem to proceed with this substrate; for example, with CNPh-DMAC-TRZ the gas chromatogram shows two additional peaks, beside the starting material and product, in comparison to the spot-to-spot conversion typically observed for the other substrates. The dehalogenation of 4-iodoanisole (Ered=−2.50 V, Figure 6f) has been reported in the literature using PTH (with the conditions previously mentioned), giving 92 % yield in 5 h under N2, or 89 % in 24 h under air.9 Under our optimized conditions, PTH yields only 14 % of the product, which is marginally better than the 7 % yield achieved with DMAC-TRZ. By contrast, a progressively increasing yield from 27 to 61 % was observed for the DMAC-TRZ derivatives, which was correlated to the Hammett constant (Figure 6f). 4CzIPN afforded similar yields to CNPh-DMAC-TRZ (58 and 61 %, respectively).
The most challenging two substrates to dehalogenate that we investigated were 3-bromoanisole (Ered=−2.59 V) and 4-bromoanisole (Ered=−2.72 V). With respect to the first substrate, low yields of 2, 9, 10 and 23 % were obtained when using 4CzIPN, dPh-DMAC-TRZ, CF3Ph-DMAC-TRZ and CNPh-DMAC-TRZ, respectively, while no product was formed with DMAC-TRZ, tBuPh-DMAC-TRZ and PTH (Figure 6g). Increasing the reaction time to 72 h resulted in a considerably increased product yield of 13, 40, 27 and 71 % using tBuPh-DMAC-TRZ, dPh-DMAC-TRZ, CF3Ph-DMAC-TRZ and CNPh-DMAC-TRZ, respectively. By contrast, the product yield increased only incrementally with 4CzIPN (10 %) while no product formed with DMAC-TRZ or PTH. The dehalogenation of 4-bromoanisole has previously been reported using [tBu−Mes−Acr]BF4 in a yield of 90 % using 10 mol % of PC, MeCN as the solvent and 16 h irradiation time under N2.10, 35 Unsurprisingly, neither DMAC-TRZ nor PTH could dehalogenate 4-bromoanisole, but each of tBuPh-DMAC-TRZ, dPh-DMAC-TRZ, CF3Ph-DMAC-TRZ and CNPh-DMAC-TRZ produced anisole in 9, 26, 18 and 30 % yield, respectively, over 72 h (Figure 6h), yields which are all equivalent to or higher than that achieved with 4CzIPN (10 %). None of the PCs tested (the DMAC-TRZ derivatives, 4CzIPN or PTH) could dehalogenate 4-chloroanisole (Ered=−2.90 V), even after 72 h, indicating a limit of the reduction capability of these PCs.
Mechanistic Investigation
Except for CNPh-DMAC-TRZ (E*ox=−1.66 V) the DMAC-TRZ derivatives tend to be equally strong photoreductants (E*ox=−1.46 V to −1.54 V) yet produced remarkably divergent yields across the substrates. Additionally, none of the PCs investigated should be thermodynamically capable of directly photoreducing the substrates, implying that these compounds cannot operate via an oxidative quenching mechanism, leading us to instead consider the conPET mechanism.8, 10, 12 For subsequent mechanistic investigation, we focused on CF3Ph-DMAC-TRZ and CNPh-DMAC-TRZ, since these tended to afford the greatest yields of dehalogenated product, as well as DMAC-TRZ as a reference comparison.
Stern–Volmer quenching studies were conducted using the model system of methyl 4-chlorobenzoate and DIPEA. To our surprise, no discernible quenching could be observed for DMAC-TRZ, CF3Ph-DMAC-TRZ or CNPh-DMAC-TRZ (Figures S16–18). This raised the question of the photostability,36 thus UV/Vis absorption spectra were measured before and after irradiation of the PC in DMF, as well as in the presence of only DIPEA and under the dehalogenation conditions of methyl 4-chlorobenzoate. The pre-irradiation profiles in all cases are reflective of the UV/Vis absorption spectrum of the PC; therefore, spectroscopic changes observed after irradiation can be assigned to changes to the structure of the PC (Figure S25–S29).
Changes in the absorption spectra were observed for all DMAC-TRZ PCs, 4CzIPN and PTH upon irradiation of the PC in DMF (Figure 7). For the DMAC-TRZ derivatives, a loss of the both the low-energy CT and LE bands were observed, causing a dramatic blue-shift of the absorption profile. Similarly, 4CzIPN showed a blue-shifted absorption spectrum, and an absence of the characteristic CT band between 350–450 nm. By contrast, PTH developed two new bands (302 and 338 nm) and the absorption onset was unaffected. After irradiation in the presence of DIPEA in DMF, changes distinct from those seen when simply irradiating the PC were observed. For DMAC-TRZ and PTH, this was manifested in the growth of a new band at ca. 415 nm. For CF3Ph-DMAC-TRZ, the absorption spectrum was like that pre-irradiation, but with a blue-shift in the LE band (357 nm) and the absorption onset. Pleasingly, CNPh-DMAC-TRZ appeared to be relatively photostable with minimal changes observed in the absorption spectrum. 4CzIPN displayed the same significant blue-shift in its absorption profile as was observed when only irradiated in DMF. Finally, the UV/Vis absorption spectra were obtained before and after irradiation under the reaction conditions of the dehalogenation of methyl 4-chlorobenzoate. 4CzIPN again displayed the same post-irradiation profile, suggesting the same photodegradation product is formed irrespective of the presence of DIPEA and methyl 4-chlorobenzoate. PTH also displayed a similar post irradiation profile to that observed after irradiation in the presence of DIPEA, implying that DIPEA plays a role in the photodegradation of PTH that forms degradation product(s) distinct from those obtained from only irradiating PTH. For CF3Ph-DMAC-TRZ and CNPh-DMAC-TRZ, and to a lesser extent, DMAC-TRZ, a red-shift in the absorption onset is observed; for CF3Ph-DMAC-TRZ and CNPh-DMAC-TRZ, the well-resolved LE band at 357 and 378 nm, respectively, becomes broad and less well defined.
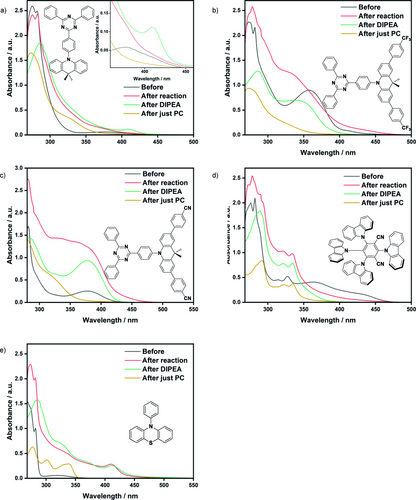
UV/Vis absorption spectra before and after irradiation in the dehalogenation of methyl 4-chlorobenzoate, and after irradiation in the presence of DIPEA and after irradiation of just the PC, all in DMF using a 390 nm Kessil lamp for 18 h. a) DMAC-TRZ, b) CF3Ph-DMAC-TRZ, c) CNPh-DMAC-TRZ, d) 4CzIPN and e) PTH. The insert in a) shows a zoomed in section of the absorption onset.
Recognising the non-innocence of DMF in the dehalogenation process, we consulted the previous photocatalysis literature employing this solvent. Using TiO2 as the PC, DMF can be photocatalytically decomposed to carbon monoxide and dimethylamine under UV light irradiation (λexc=365 nm).37 In the presence of water vapour, using a metal-organic-framework PC and visible light irradiation (26 W helical light bulb), the decomposition products of DMF were instead proposed to be formic acid and dimethylamine.38, 39 It is clear that under certain conditions, DMF is not a benign solvent for photocatalysis reactions. We thus attempted to understand its influence on the photodegradation of model PCs 4CzIPN and DMAC-TRZ.
To isolate the photodegradation products, 4CzIPN was irradiated in DMF for 18 h at five times the reaction scale, which led to complete depletion of 4CzIPN (Figure S37 and S42). Following purification attempts, carbazole was isolated, as confirmed by 1H NMR and GC-MS (Figures S38, S40 and S41); further, the UV/Vis absorption spectrum of carbazole in DMF mirrors quite closely the post-irradiation UV/Vis absorption spectrum of 4CzIPN (Figure S31). The GC-MS shows an additional peak with m/z of 195 (Figure S40 and S41). Based on this mass and on the 1H NMR spectrum (Figure S39), we tentatively assigned the identity of this species as N-formylcarbazole based on a cross-comparison with the spectrum from the literature.40 Other products could neither be cleanly isolated nor identified.
A similar experiment was conducted with DMAC-TRZ (i.e. irradiation at five times the reaction scale); however, no photodegradation was observed by UV/Vis absorption spectroscopy. To interrogate this discrepancy in behaviour, steady-state PL spectra were obtained for the post-irradiation profile of DMAC-TRZ irradiated in DMF at the scale used in the dehalogenation reaction and at five times the scale (Figure 8). After irradiation at normal scale, the emission is considerably blue-shifted (λPL=486 nm compared to 655 nm for DMAC-TRZ in DMF), while the post-irradiation profile conducted at five times the normal scale shows an emission spectrum that is largely reflective of DMAC-TRZ; a small shoulder is observed between 400–500 nm. After irradiation of methyl 4-chlorobenzoate in the presence of DIPEA, using DMAC-TRZ as the PC, both at the normal reaction scale and five times the normal reaction scale, the emission profiles are almost identical, with λPL of 455 nm (Figure 8). This indicates that irrespective of the scale used, DMAC-TRZ photodegrades consistently in the dehalogenation reaction, and that this degradation is distinct to that observed when DMAC-TRZ is photoexcited in DMF. These degradation products are not DMAC or triazine, based on a cross-comparison of their respective emission spectra (Figure 8).
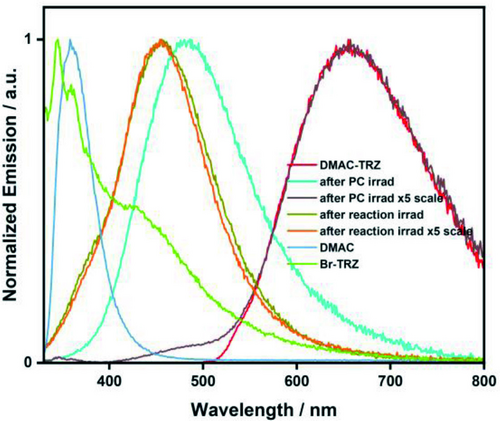
Normalized steady-state PL of DMAC-TRZ in DMF and of DMAC-TRZ after irradiation in DMF (at normal reaction scale and 5× the normal scale) as well as the post-irradiation profile of the reaction mixture (methyl 4-chlorobenzoate, DIPEA, DMAC-TRZ in DMF) at normal scale and at 5× the normal scale. All irradiation conducted with a 390 nm Kessil LED excitation source. For PL spectra, λexc=290 nm.
These studies lead us to conclude that the family of DMAC-TRZ derivatives photodegrades into species that are sufficiently photoreducing to promote the dehalogenation of the aryl halides; we were unable to either isolate or characterize these photodegradation products. The poor photostability observed for all PCs investigated, including literature PCs, raises the question of whether these compounds can be classified as PCs, or whether they are better described as pre(photo)catalysts.
Conclusion
A series of five donor-acceptor TADF compounds were developed to target the photoreduction of aryl halides. Modification of the donor group of the parent compound DMAC-TRZ with a Hammett series of p-substituted phenyl moieties, resulted in significant changes to the optoelectronic properties. For example, Eox varied from 0.97 V for the parent compound, to 0.79–1.04 V, depending on whether the donor was substituted with an aryl substituent bearing an EDG or EWG. Consequently, E*ox ranged from −1.46 V to −1.66 V, with the most photoreducing derivative, CNPh-DMAC-TRZ (E*ox=−1.66 V), comparable to fac-Ir(ppy)3 (E*ox=−1.70 V).
All five of the substituted DMAC-TRZ derivatives proved to be more potent PCs than the parent compound and could dehalogenate aryl halides with Ered ranging from −1.88 to −2.72 V vs SCE. Particularly, CF3Ph-DMAC-TRZ and CNPh-DMAC-TRZ provided the highest yields, with the latter achieving yields of 30–71 % for substrates with Ered < −2.5 V. Although these yields are lower than those reported for similar substrates using [tBu−Mes−Acr]BF4,10 our photocatalysis conditions proceed under air and at lower PC loading (1 mol %). Mechanistically, however, all PCs photodegrade under the reaction conditions, with the resultant photodegradation product responsible for the required dehalogenation.
Supporting Information
The authors have cited additional references within the Supporting Information.41-43
Acknowledgments
We are grateful to the University of St Andrews, Syngenta, and the EPSRC Centre for Doctoral Training in Critical Resource Catalysis (CRITICAT) for financial support [Ph.D. studentship to “M.A.B.”; Grant code: EP/L016419/1]. E.Z.-C. acknowledges EPSRC for support (EP/W007517/1). This project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska Curie grant agreement No 812872 (TADFlife). We are thankful to the SFC Saltire Emerging Researcher Scheme for funding the placement of M.A.B to the A.S. lab. We thank Umicore AG for the gift of materials.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The research data supporting this publication can be accessed at https://doi.org/10.17630/35de1560-7cbc-458b-91f4-9f051e193e37