Photo-Responsive Hydrogen-Bonded Molecular Networks Capable of Retaining Crystalline Periodicity after Isomerization
Graphical Abstract
Abstract
The molecular conformation, crystalline morphology, and properties of photochromic organic crystals can be controlled through photoirradiation, making them promising candidates for functional organic materials. However, photochromic porous molecular crystals with a networked framework structure are rare due to the difficulty in maintaining space that allows for photo-induced molecular motion in the crystalline state. This study describes a photo-responsive single crystal based on hydrogen-bonded (H-bonded) network of dihydrodimethylbenzo[e]pyrene derivative 4BDHP. A crystal composed of H-bonded undulate layers, 4BDHP-2, underwent photo-isomerization in the crystalline state due to loose stacking of the layers. Particularly, enantio-pure crystal (S,S)-4BDHP-2 allowed to reveal the structure of the photoisomerized crystal, in which the closed form (4BDHP) and open form (4CPD) were arranged alternately with keeping crystalline periodicity, although side reactions were also implied. The present proof-of-concept system of a photochromic framework that retains crystalline periodicity after photo-isomerization may provide new light-driven porous functional materials.
Introduction
The molecular conformation, crystalline morphology, and properties of photochromic organic crystals can be controlled spatiotemporally through photoirradiation.1, 2 Crystals composed of molecules with photochromic moieties, such as azobenzene,3-5 diarylethene,6 and spiropyrane,7, 8 have been investigated intensively for potential applications to optical memory, switches, surface modulators, and actuators.6, 9-13 The importance of photo-responsive framework materials is growing because they can be applied to materials capable of selective adsorption and desorption, susceptive sensors, and molecular machines allowing controllable motion of certain parts.14 Materials exhibiting successful photo-responsiveness are mainly based on metal–organic frameworks (MOFs)15 incorporating photochromic moieties in the backbone of the framework16-18 and in pendant side chains.19 The MOFs accommodating photochromic guest molecules in their inner spaces also show photo-responsive behavior.20 Recently, photo-switchable covalent-organic frameworks (COFs) based on photochromic molecules have also been reported.21, 22
Molecular crystalline frameworks constructed through non-covalent interactions, called noncovalent organic frameworks (nCOFs), are suitable systems for examining fundamental structure–property relationships because they can be isolated as single crystals suitable for X-ray diffraction analysis.23-27 When well-defined directional H-bonds are used for framework construction, the resulting H-bonded organic framework (HOF)28 often gives a predictable crystal structure.29-38 However, photo-responsive nCOFs or HOFs are still rare, although a HOF including photo-switchable guest molecules was reported.39 This is probably because HOFs tend to form more densely-packed frameworks than MOFs and COFs, resulting in no structural tolerance for photochromic molecular motions. More importantly, such non-covalent frameworks change their structural periodicity and crystallinity, even if a photochromic reaction occurs. Baroncini, Comotti, Grepioni and Credi et al. reported that a star-shaped azobenzene tetramer underwent transformation from a porous crystal to a non-porous amorphous melt phase through E-to-Z photo-isomerization.40 Wang and Jiang et al. reported that a molecular crystal composed of a diarylethene-incorporated molecular cage underwent photo-isomerization, producing a different crystalline phase with an unknown crystal structure.41
This study designed a structurally predictable H-bonded network that could undergo photo-isomerization while maintaining crystalline periodicity (Figure 1a). Firstly, a dihydrodimethylpyrene (DHP) core42-44 was applied as the photochromic moiety based on the hypothesis that the network structure connected at 4,5,9,10-positions of DHP can retain crystalline periodicity during photo-isomerization (Figure 1b). The resultant H-bonded crystal, however, showed no photo-isomerization, probably due to the low quantum yield of the ring opening reaction.45 Subsequently, a dihydrodimethylbenzo[e]pyrene (BDHP) core46 exhibiting the higher quantum efficiency for the ring opening reaction was applied for the network construction (Figure 1c).47
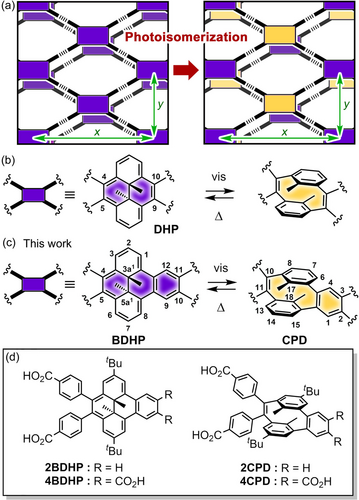
(a) Concept of photo-responsive hydrogen-bonded frameworks that undergo a photochromic reaction while maintaining crystallinity and structural periodicity. Photo-isomerization of (b) dimethyldihydropyrene (DHP) and (c) dimethyldihydrobenzo[e]pyrene (BDHP). The H-bonded sql-network composed of 4BDHP is expected to retain structural periodicity (x and y directions) upon isomerization to 4CPD. (d) Carboxylic acid derivatives used in the study.
This report describes, for the first time, a proof-of-concept system of photo-responsive H-bonded crystalline framework capable of retaining crystalline periodicity after photo-isomerization, although the permanent porosity has not been achieved yet. The network was composed of 4BDHP, which isomerizes into 4CPD by visible light irradiation. We synthesized tetracarboxylic acid derivative 4BDHP and dicarboxylic acid analog 2BDHP (Figure 1d), prepared their H-bonded network crystals [4BDHP-1, 4BDHP-2, and 2BDHP-1], and investigated their photo-isomerization behavior in solution and crystalline states. Crystal 4BDHP-2 and its enantiopure form (S,S)-4BDHP-2 with undulate sql-topological network structures underwent photo-isomerization by visible light irradiation, although photoirradiation also induced side reactions. Remarkably, we succeeded in analyzing the crystal structure of photo-isomerized (S,S)-4BDHP-2. The resultant crystal (S,S)-4BDHP-2-Vis kept H-bonded crystalline network, and the photo-isomerized molecule 4CPD and non-isomerized molecule 4BDHP were alternately arranged in the network. These results open the door to creating new types of photo-responsive porous materials.
Results and Discussion
Synthesis of Compounds
Dibromodihydrodimethylbenzo[e]pyrene derivative 2 was synthesized from pristine DHP according to a previous report.48 Suzuki–Miyaura cross-coupling reaction of 2 and 3 gave dimethyl ester derivative 4, which was hydrolyzed in the presence of KOH to give 2BDHP (Scheme 1a). Similarly, dimethyl ester derivative 5, which was prepared according to the reported method,49 was brominated with N-bromosuccinimide, and the resultant dibromide 6 was reacted with 3 to give tetramethyl ester derivative 7 (Scheme 1b). Hydrolysis of 7 in the presence of KOH gave 4BDHP. The synthesized compounds were characterized by 1H and 13C NMR spectroscopy and HR-MS. Both 2BDHP and 4BDHP were obtained as racemic mixtures of (R,R)- and (S,S)-enantiomers (no specific symbol is attached for the racemic mixture in this report).
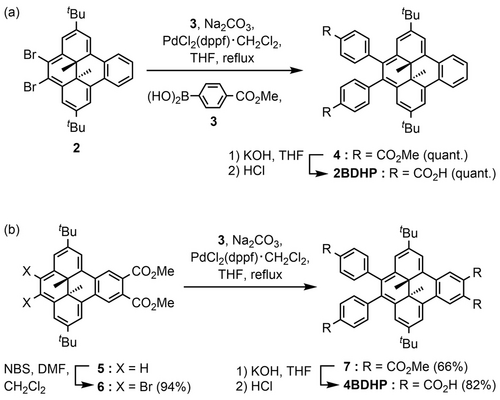
Synthesis of (a) 2BDHP and (b) 4BDHP.
Theoretical Calculations
Geometric optimization of 4BDHP and 4CPD was performed at the B3LYP/6-311+G** level of theory (Figure S1). Figure 2 shows superimposed molecular conformations of 4BDHP and 4CPD. Characteristic structural parameters are listed in Table 1. 4BDHP contains the C(3a1)−C(5a1) single bond with a bond length (d) of 1.543 Å, while the corresponding bond is cleaved in 4CPD: the distance C(17)⋅⋅⋅C(18) is 2.688 Å. The C−C bond length (dMe) between the inner methyl carbon and C(3a1) or C(5a1) in 4BDHP (i.e., 1.569 Å) is slightly longer than the corresponding distance in 4CPD (i.e., 1.506 Å).
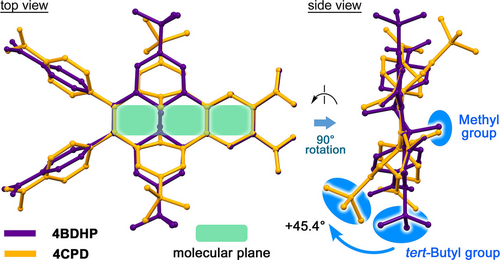
Superimposed molecular structures of 4BDHP (purple) and 4CPD (orange) geometrically optimized by DFT calculations at the B3LYP/6-311+G** level of theory.
Molecule |
d[a]/Å |
dMe[b]/Å |
ωC[c]/° |
ωCP[d]/° |
ωtB[e]/° |
|---|---|---|---|---|---|
Optimized 4CPD |
2.688 |
1.506 |
37.19, 37.46 |
50.72, 50.91 |
47.9 |
Optimized 4BDHP |
1.543 |
1.569 |
35.90, 35.96 |
81.28, 81.78 |
2.5[f] |
A in (S,S)-4BDHP-1 |
1.504 |
1.554, 1.537 |
44.77, 44.87 |
59.03, 59.19 |
6.1 |
B in (S,S)-4BDHP-1 |
1.491 |
1.548, 1.553 |
46.25, 46.69 |
60.66, 60.35 |
–[g] |
A in (S,S)-4BDHP-2 |
1.602 |
1.551 |
46.17 |
83.89 |
15.1 |
B in (S,S)-4BDHP-2 |
1.472 |
1.610 |
44.14 |
86.20 |
7.3 |
A in (S,S)-4BDHP-2-Vis-7h |
2.345 |
1.473 |
59.56 |
66.88 |
22.4 |
B in (S,S)-4BDHP-2-Vis-7h |
1.551 |
1.552 |
43.78 |
69.57 |
−10.7 |
- [a] C(3a1)−C(5a1) bond length for 4BDHP or C(17)⋅⋅⋅C(18) distance for 4CPD. [b] C(3a1)−CH3 or C(5a1)−CH3 bond length for 4BDHP or C(17)−CH3 or C(18)−CH3 bond length for 4CPD. [c] Dihedral angles between the mean plane of the molecule defined by C(4), C(5), C(10), and C(11) atoms for 4BDHP or C(2), C(3), C(10), and C(11) atoms for 4CPD, and the mean plane of the carboxy group. [d] Dihedral angles between the mean plane of the molecule and the mean plane of the peripheral carboxyphenyl group. [e] Dihedral angles between the mean plane of the molecule and the mean plane of the tert-butyl group defined by C(1), C(2), C(3), and C(Me)3 atoms for 4BDHP or C(6), C(7), C(8), and C(Me)3 atoms for 4CPD. [f] Angle between the mean plane of the molecule and the C(2)−C(Me)3 bond. [g] Values are negligible.
The benzene rings of the cyclophane moiety in 4CPD are slightly bent because of bridging at the meta-position of the ring: the root mean square deviation (RMSD) of the rings is 0.080 Å. Note that the peripheral tert-butyl groups in 4CPD are significantly bent up and down from the molecular plane (ωtB: ca. 2.5° for 4BDHP and 47.9° for 4CPD), instead of the closely similar positions for the methyl groups in 4BDHP and 4CPD. This comparison also shows differences in the rotational conformation of the peripheral carboxyphenyl groups (ωCP: 81.28–81.78° for 4BDHP and 50.72–50.91° for 4CPD) and carboxy groups (ωC: 35.90–35.96° for 4BDHP and 37.19–37.46° for 4CPD). The TD-DFT calculations revealed that 4BDHP has lower LUMO and higher HOMO levels and a smaller HOMO–LUMO gap (−2.69 eV, −5.25 eV, and 2.56 eV, respectively) compared with the corresponding values for 4CPD (−2.46, −5.81, and 3.35 eV, respectively) due to the π-conjugated benzo[14]annulene rings of 4BDHP (Figure S2). The HOMO and LUMO of 4BDHP are delocalized throughout the BDHP skeleton, while those in 4CPD are located mainly on the cyclophane and peripheral phenylene rings. The 2BDHP and 2CPD show a similar tendency (Figure S3).
Photo-isomerization in Solution
Photo-isomerization of the BDHP derivatives was first examined in a solution state. 2BDHP and 4BDHP were subjected to irradiation with visible light (λ>430 nm) using a xenon lamp in DMF solutions, and isomerization reactions were monitored by UV/Vis and 1H NMR spectroscopy. Figure 3a shows UV/Vis spectral changes of 4BDHP upon irradiation. 4BDHP produced absorption bands at 355 and 410 nm with shoulders and a broad band ranging from 450 to 650 nm. Upon photoirradiation, the absorption bands characteristic of 4BDHP gradually decayed and reached a steady state in 80 s, while a new band at 266 nm became prominent. These spectral changes agree well with the theoretical electronic transition calculated for 4BDHP and 4CPD (Figure 3b), indicating that the expected photo-isomerization occurred. Thermal treatment at 80 °C for 15 min prompted the ring closing reaction to give the original spectral profile. The UV/Vis spectral changes gave the isosbestic point at 315 nm, showing clean photo-isomerization without side reactions. The 2BDHP also showed similar behavior (Figure S4).
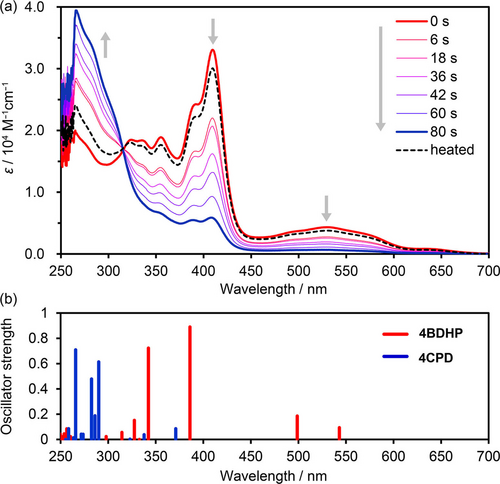
(a) UV/Vis spectral changes of 4BDHP upon visible light irradiation in a DMF solution (1.74×10−5 M). The original spectral profile was nearly recovered after heating at 80 °C for 15 min, as shown by the dotted line. (b) Oscillator strengths of electronic transition for 4BDHP (red) and 4CPD (blue) calculated at the TD-CAM-B3LYP/6-311+G** level of theory with PCM(DMF).
Thermally induced ring closing reactions of 4CPD and 2CPD in DMF solutions were monitored by UV/Vis spectroscopy at various temperatures (Figures S5–S8), which revealed that the activation energy (Ea) of the ring closing reaction was 95.0 kJ/mol for 2CPD and 106 kJ/mol for 4CPD. These data agree well with those for other CPD derivatives reported in the literature.50 The half-life periods of 4CPD and 2CPD at 40 °C were approximately 4 and 3 hours, respectively, and those at 70 °C were 5 and 10 min, respectively (Tables S1 and S2).
Figure 4 shows 1H NMR spectral changes of 4BDHP upon irradiation and subsequent heating in the dark. A peak ascribable to protons of the inner methyl groups in 4BDHP resonated at −1.53 ppm almost disappeared after visible light irradiation, while a peak of the corresponding benzyl protons of 4CPD appeared at 1.46 ppm. Because of loss of aromaticity of the [14]annulene ring, signals associated with aromatic protons in the annelated benzene ring at 9.33 ppm and those for the [14]annulene ring at 8.66 and 7.21 ppm shifted up-field to 7.98, 6.87, and 6.44 ppm, respectively. Similarly, the resonance of tert-butyl protons shifted up-field from 1.34 to 1.06 ppm upon irradiation. After heating at 80 °C for 1 h, the original spectrum was recovered. The spectral changes also provided information about the rotational mobility of the peripheral phenylene rings. In the spectrum of 4BDHP, the aromatic protons of the phenylene rings resonated at four different positions of 7.87, 7.79, 7.43, and 7.16 ppm as broad doublets, while two sets of sharp doublets at 7.78 and 7.21 ppm were observed in the spectrum of 4CPD, indicating that the mobility of the phenylene rings is highly restricted in 4BDHP due to steric hindrance of the nearly planar [14]annulene ring. 1H NMR spectral changes for 2BDHP upon visible light irradiation showed similar results (Figure S9).
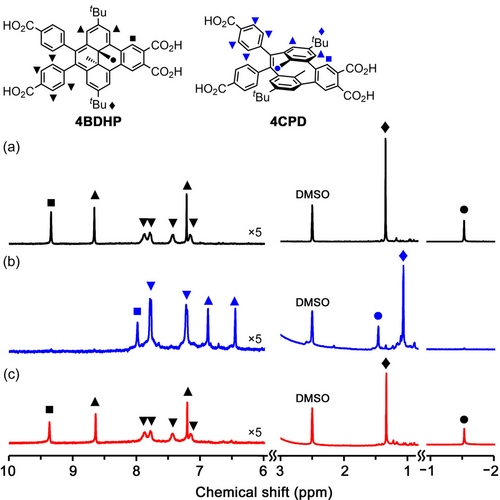
1H NMR spectral changes of 4BDHP upon photo-isomerization with visible light irradiation in a DMSO-d6 solution (4.80×10−3 M). (a) Before irradiation, (b) after irradiation for 5 min at room temperature, and (c) after heating the irradiated solution for 1 h at 80 °C. Height of the spectra was modified for clarity.
Crystallography and Photo-isomerization of 2BDHP
First, pristine BDHP was recrystallized from a THF solution at room temperature, resulting in precipitation of single crystals with Pbca space group (Figure S10, Table S3). Visible light irradiation of the crystal resulted in no color changes but did increase the roughness of the crystal surface (Figure S11). Next, dicarboxylic acid derivative 2BDHP was recrystallized using a mixed solution of DMF and methyl benzoate (MeBz) at 90 °C or using a mixed solution of THF and MeBz at room temperature, where MeBz was used as a template molecule, giving single crystals suitable for X-ray analysis (Table S3). 2BDHP crystallized in space group P21/c with inclusion of MeBz molecules, giving crystal 2BDHP-1. The inner methyl groups and carbon atoms C(5a1) and C(3a1) of 2BDHP are disordered in two sites (Figure 5a). Molecules of 2BDHP form a zigzag-shaped 1D ribbon with a periodicity of 15.8386(4) Å along the b axis through intermolecular H-bonds between the carboxy groups (Figure 5b). The 1D ribbons are stacked to form a layered structure (Figure S12). The crystals in the mother liquid were exposed to visible light, resulting gradually fragmentation into small particles from the edge of the crystal, along with a color change from dark red to brown. Furthermore, yellow powders also precipitated from the liquid during the irradiation. Fortunately, a single orange crystal of relatively larger size and better quality was obtained by chance, allowing a preliminary crystallographic analysis of the open form contained crystal 2BDHP-X. In the crystal, the distance between C(3a1) and C(5a1) atoms is 1.85 Å, which is much longer than that in the closed form 2BDHP (1.53 Å) (Figure 5c). This observation indicated a contribution of 2CPD species coexisting in the crystal. The molecules also formed a zigzag-shaped 1D ribbon through H-bonds (Figures 5d, S14). However, its periodicity, i.e. 19.822(5) Å, is much longer than that in 2BDHP-1. These structural differences imply that isomerization from 2BDHP to 2CPD in a single crystalline state may be difficult due to the incongruence of periodicities of the H-bonded 1D structures.
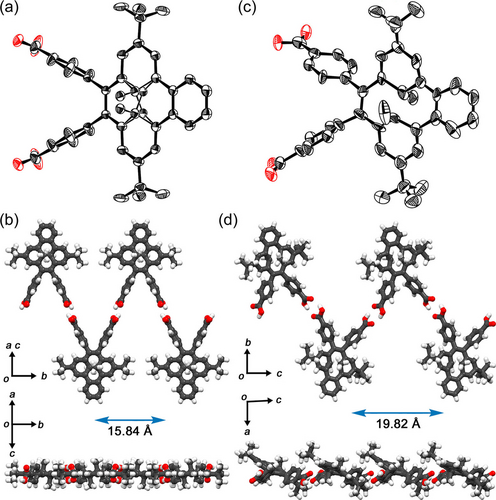
Crystal structures of (a, b) 2BDHP-1 and (c, d) 2BDHP-X. Anisotropic displacement ellipsoid plots of (a) 2BDHP-1 and (b) 2BDHP-X are drawn with 50 % and 20 % probabilities, respectively. The inner methyl groups are disordered in 2BDHP-1. Top and side views of the H-bonded zig-zag ribbon motifs of (b) 2BDHP-1 and (d) 2BDHP-X.
Crystallography and Photo-isomerization of 4BDHP
4BDHP was crystallized by slow evaporation from a mixed solution of DMF and MeBz at 90 °C, yielding monoclinic P21/c crystal 4BDHP-1 (Figure 6a–d, Table S4). The inner methyl groups and carbon atoms C(3a1) and C(5a1) of 4BDHP are disordered in two sites with a chemical occupancy of 0.64 and 0.36. The 4BDHP forms a sql-networked flat sheet through intermolecular H-bonds of the carboxy groups. The network has periodicities of 33.18 Å and 15.25 Å along the b axis and ac diagonal axis directions, respectively. This type of sql-network is typical for tetracarboxylic acid tectons with rigid planar skeletons.51, 52 Neighboring tert-butyl groups divide the rhombic void into two triangular voids with a side length of ca. 8.2 Å. The solvent-accessible volume was estimated to be 13 % by PLATON software using a probe radius of 1.2 Å.53 In the void, molecules of MeBz are accommodated with a 1 : 2 host–guest ratio. The solvent molecules were fixed through π/π stacking between the carboxy dimer and the benzene ring of MeBz and multiple CH/O contacts (Figure S15). Molecules with the same chemical occupancy constitute one sql-networked sheet, and therefore chirality of the sheet slightly leaned to one side, such as the (S,S)-rich sheet. The layers are, however, stacked in an inverted manner, resulting in the achiral layered framework of 4BDHP-1. The layers are stacked with an interlayer distance of 5.08 Å through contacts between the methyl groups and the [14]annulene ring as well as CH/O contacts of the carbonyl oxygen atoms and the aromatic hydrogen atoms (Figure S16).
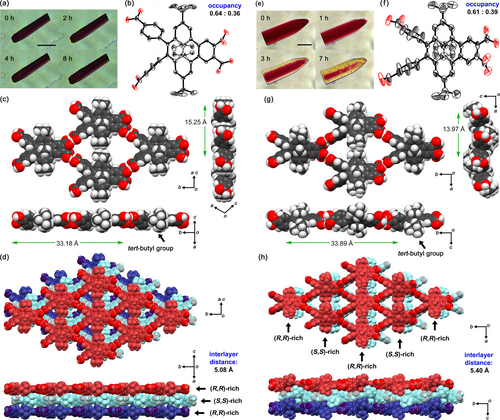
Crystal structures of (a-d) 4BDHP-1 and (e-h) 4BDHP-2. (a, e) Photographs of single crystals during visible light irradiation (scale bar: 50 μm). (b, f) Molecular structures drawn in anisotropic displacement ellipsoid plots with 50 % probability, where one tert-butyl group in (b) is disordered. The inner methyl groups, and carbon atoms C(3a1) and C(5a1) in (b) and (d) are also disordered with occupancies of 0.64/0.36 and 0.61/0.39, respectively. (c, g) H-bonded sql-network sheet motifs with structural periodicities. (d, h) Stack of three layers. Minor portions of the disordered structures are omitted in (c, d, g, h) for clarity.
Meanwhile, crystallization from a mixed solution of DMF and 1,2-dimethoxybenzene (DMB) at 90 °C yielded orthorhombic Pbcn crystal 4BDHP-2 (Figure 6e–h). The molecule has a two-fold axis. The inner methyl groups and carbon atoms at C(3a1) and C(5a1) of 4BDHP are disordered in two sites with chemical occupancies of 0.61 and 0.39. The 4BDHP forms a sql-networked undulate sheet parallel to the ab plane through intermolecular H-bonds of the carboxy groups. The network has periodicity of 33.89 Å and 13.97 Å along the b- and a-axis directions, respectively. In the sheet, R,R-rich and S,S-rich molecules are alternately connected through H-bonds with opposite inclines of 18.4° against the ab plane (Figures 6g and S17). Because of the incline, the tert-butyl groups are out of the plane. The undulate layers are stacked in an inverted manner with interlayer distances of 5.47 Å through contacts between the methyl groups and the annelated benzene ring as well as CH/O contacts of the carbonyl oxygen atoms and the aromatic hydrogen atoms (Figure S18). Molecules of DMB in the inclusion space of 4BDHP-2 were severely disordered and were not able to be refined crystallographically. The void ratio of the inclusion space was 10 %.
Thermal analyses of 4BDHP-2 revealed that the crystallinity was lost by heating up to ca. 220 °C due to removal of included DMB molecules and thermal decomposition of the molecules themselves (Figures S19–S21). 1H NMR spectrum of the decomposed compounds indicates that the thermal decomposition was proceeded by 1,5-sigmatropic shift of the inner methyl groups and irreversible cascade reactions (Figure S22), which was reported on DHP derivatives.54 These indicate thermal limitation of the present systems.
Crystals of 4BDHP-1 were subjected to visible light irradiation for 8 h and showed no changes in either appearance (Figure 6a) or crystal structure (Table S5, Figure S23) due to tight packing of the molecules in the crystals. In contrast, crystals of 4BDHP-2 underwent color changes from dark red to yellowish red accompanied by generation of cracks upon irradiation (Figure 6e). This indicates that 4BDHP-2 undergoes photo-isomerization due to loose packing of the undulate layers. Single-crystalline X-ray diffraction experiments revealed a structure of a photoirradiated crystal containing 4BDHP with an elongated C(5a1)−C(3a1) bond (Table S5 and Figure S23). Further irradiation, however, resulted in loss of single-crystallinity. 1H NMR spectrum of the well-irradiated crystal dissolved in DMSO-d6 indicated that side reactions also might occur (Figure S25). Since 4BDHP-2 is composed of disordered racemic 4BDHP molecules, photoisomerized crystals can contain (R,R)-4BDHP, (S,S)-4BDHP, (Rp)-4CPD, and (Sp)-4CPD. Particularly, the coexistence of (Rp)-4CPD and (Sp)-4CPD must disturb the crystallinity because of their prominent structural differences. Based on this hypothesis, the enantiomers of 4BDHP were separated.
Enantiomer Separation of 4BDHP
Resolution of 4BDHP was conducted as shown in Figure 7. As described earlier, the BDHP and CPD derivatives are chiral compounds. Two chiral centers at C(3a1) and C(5a1) in 4BDHP results in (S,S)- and (R,R)-isomers. Similarly, 4CPD has planar chirality.55, 56 The definition of planar chirality in 4CPD is shown in Figure S26. The (Sp)-4CPD and (Rp)-4CPD can be provided by ring opening reactions of (S,S)-4BDHP and (R,R)-4BDHP, respectively.
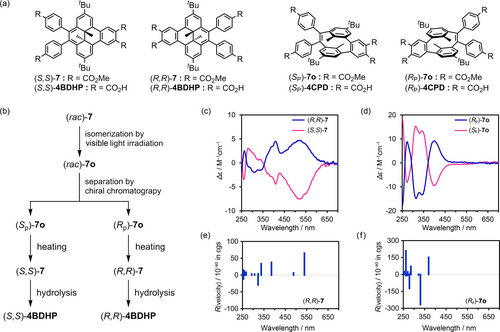
Resolution of (rac)-7. (a) Enantiomers of BDHP and CPD derivatives. (b) Procedure of the resolution. Experimentally observed CD spectra of enantiomers (c) 7 and (d) 7 o in chloroform solutions. Theoretical ECD of (e) (R,R)-7 and (f) (Rp)-7 o calculated at TD CAM-B3LYP/6-311+G** level of theory with PCM(CHCl3).
The racemic mixture of methyl ester precursor (rac)-7 dissolved in chloroform was irradiated with a white LED light until (rac)-7 was isomerized into (rac)-7 o. The resultant solution was immediately subjected to preparative HPLC equipped with a chiral column (Figures S27, S28), allowing isolation of enantiomers (Sp)-7 o and (Rp)-7 o with 99.1 %ee and 94.9 %ee, respectively (Figures S29 and S30). The enantiomers also were confirmed to isomerize into the corresponding BDHP isomer upon heating (Figure S31 and S32). Optical properties of the separated enantiomers (Sp)-7 o and (Rp)-7 o and the corresponding BDHP isomers (S,S)-7 and (R,R)-7 are shown in Figure 7c and 7d, respectively. The absolute structures of the enantiomers of 7 and 7o were assigned by comparison with the theoretical CD signals calculated for each of the enantiomers (Figure 7e,f). The enantiomers of 7 showed CD bands in the range of 450–650 nm, which corresponds to the HOMO–LUMO transition, although the intensity of the CD spectra was weak because of subtle differences in the structures. These bands are characteristic of BDHP and were also predicted by the theoretical calculation. The CD spectra of 7 o show prominent bands at 273, 314, 343, and 402 nm. The separated (Sp)-7 o and (Rp)-7 o were isomerized back to (S,S)-7 and (R,R)-7, respectively, and subjected to hydrolysis, giving (S,S)-4BDHP and (R,R)-4BDHP, respectively, which were used for construction of enantiopure H-bonded framework. The absolute structure of enantio-pure 4BDHP was also confirmed by X-ray crystallography with Cu−Kα X-ray source (vide infra).
Structure and Photo-isomerization of Enantiopure Crystal
Crystallization of (S,S)-4BDHP from a mixed solution of DMF and MeBz gave monoclinic P21 crystal (S,S)-4BDHP-1 (Table S6, Figure S33). The crystal had a molecular arrangement closely similar to that in racemic 4BDHP-1 (Figure S34). Crystallization of (S,S)-4BDHP from a mixed solution of DMF and DMB yielded orthorhombic P21212 crystal (S,S)-4BDHP-2 (Figure 8a). The crystal is composed of crystallographically independent two (S,S)-4BDHP molecules (A and B) (Figure 8b). (S,S)-4BDHP-2 also has a structure closely similar to that of racemic 4BDHP-2. Molecules A and B are connected alternately through H-bonds of the carboxy groups to form a sql-networked undulate layer (Figure 8c). The molecules A and B are inclined by 15.4° and 20.5°, respectively, against the bc plane (Figure S35). The undulate layers are stacked with interlayer distances of 5.43 Å through interlayer contacts similar to those in the racemic crystal. Further detailed structural comparison revealed that the relative positions of the inner methyl groups and the dihedral angle between the peripheral phenylene rings were different in the racemic and enantiopure crystals (Figure S36). Molecules of DMB in voids of the framework were severely disordered.
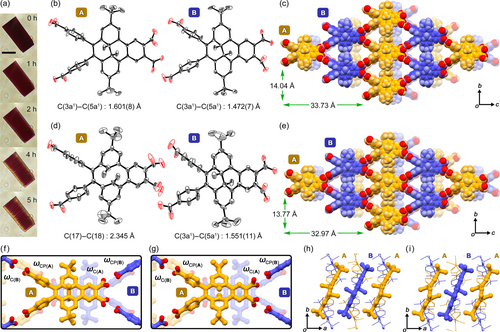
Photo-isomerization of a single crystal of (S,S)-4BDHP-2. (a) Color changes of the crystal upon irradiation (scale bar: 50 μm). Structure of (b, c, f, h) crystal (S,S)-4BDHP-2 and (d, e, g, i) crystal photo-irradiated for 7 h (S,S)-4BDHP-2-Vis-7h. (b,d) Two crystallographically independent molecules A and B drawn as anisotropic displacement ellipsoids with 20 % probability. For (S,S)-4BDHP-2-Vis-7h in (d), molecule A is contributed by the configuration of (Sp)-4CPD, while B by (S,S)-4BDHP-2. (c, e) Packing diagrams, where molecules colored orange and purple correspond to molecules A and B, respectively. (f, g) Dihedral angles of the carboxy and carboxy phenyl groups. (h, i) Alignment of the molecular cores of molecules A (orange) and B (purple).
Interestingly, one of the independent molecules (molecule A) has a longer C(3a1)−C(5a1) bond with a length of 1.601(8) Å, while that of molecule B shows a typical length of 1.472(7) Å. This implies that the photo-isomerization of 4BDHP may start even under an ambient condition before affirmative irradiation. Indeed, X-ray diffraction analyses, performed on four different single crystals of (S,S)-4BDHP-2 (Table S7), revealed that the C(3a1)−C(5a1) bond length in molecule A varied from 1.565(4) Å to 1.726(14) Å (Table S8), although the crystals had the same space group, i.e. P21212, and the same networked structures (Figure S37).
Visible light irradiation to a single crystal of (S,S)-4BDHP-2 changed the color of the crystal from dark red to brown (Figure 8a). Simultaneously, cracks were generated parallel to the (100) face, on which the H-bonded sql-network sheets are laid. Single crystal X-ray diffraction analysis successfully revealed a structure of the crystal irradiated for 7 h, (S,S)-4BDHP-2-Vis-7h (Figure 8d, e). Surprisingly, only molecule A changes its structure into a chair-like configuration corresponding to (Sp)-4CPD. The C(3a1)−C(5a1) bond was cleaved: the distance between carbon atoms C(17) and C(18) was 2.345 Å. In addition, the two peripheral tert-butyl groups were bent up and down by 33° compared with those of 4BDHP. Molecule B, in contrast, shows the same structure with that before irradiation. However, the well-organized alternate arrangement of molecules A and B strongly indicates that both molecules A and B experienced reversible ring opening and closing reactions, and consequently reached a thermodynamically stable steady state to yield (S,S)-4BDHP-2-Vis-7h. Otherwise, the photo-isomerization reaction would have occurred in a random manner, resulting in one averaged molecular structure.
Further structural analysis revealed the following structural changes. Surface shapes of the inclusion channel in the crystals changed upon the isomerization (Figure S38). The dihedral angles of the carboxy groups ωC(A) and carboxyphenyl groups ωCP(A) of molecule A against the molecular plane changed from 46.17° to 59.56° and from 83.89° to 66.88°, respectively, upon the isomerization (Table 1, Figure 8f, g). Similarly, the corresponding angles of molecule B, ωC(B) and ωCP(B) changed from 44.14° to 43.78° and from 86.20° to 69.57°, respectively. Additionally, only molecule A inclined by ca. 11° from the bc plane after the isomerization to maintain the approximate position of the bulky tert-butyl groups in the alternating alignment of molecules A and B (Figures 8h, i and S39). The distance between the quaternary carbon atoms of the tert-butyl groups of neighboring molecules A and B is 6.480 Å in (S,S)-4BDHP-2, and is 6.410 Å in (S,S)-4BDHP-2-Vis-7h. These structural adjustments help prevent changes in the framework periodicity and maintain crystallinity of the frameworks.
It is remarkable that molecule A in (S,S)-4BDHP-2-Vis-7h has an intermediate conformation between 4BDHP and geometrically optimized 4CPD, as shown in Figure 9. When these three structures are superimposed to match the molecular plane defined by C2, C3, C10, and C11, the positions of the inner methyl groups are approximately the same in all three structures. However, the positions of the tert-butyl groups are significantly different among the molecules due to bending caused by the isomerization. The bending angle of the ideal conformation of 4CPD was 47.9°, while that of molecule A in (S,S)-4BDHP-2-Vis-7h remained at 22.4°. Although the molecule A was also solved by a disordering model with superimposed 4CPD and 4BDHP (Figure S40, Table S10), the conformation of 4CPD remains the intermediate form. The differences between the theoretical and observed conformation of 4CPD could be attributed to the fact that molecule A was disrupted to accommodate the most stable conformation of 4CPD due to steric hindrance in the framework.
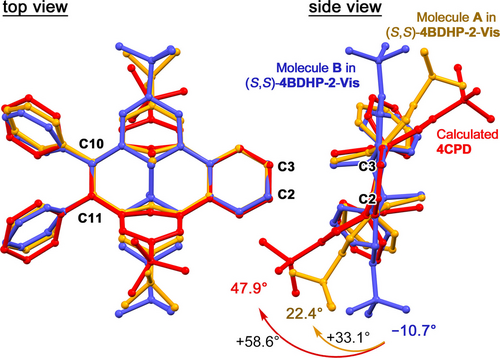
Conformational and configurational comparison of molecules A (blue) and B (orange) in (S,S)-4BDHP-2-Vis-7h and the calculated structure of 4CPD (red). The structures are superimposed to overlap the molecular plane defined by C(2), C(3), C(10), and C(11) atoms. Carboxy groups were omitted for clarity.
Other crystals irradiated by visible light for 4 h [(S,S)-4BDHP-2-Vis-4h] and for 14 h [(S,S)-4BDHP-2-Vis-14h] were also analyzed. (S,S)-4BDHP-2-Vis-4h has lower crystallinity than (S,S)-4BDHP-2-Vis-7h, and has the shorter C(17)⋅⋅⋅C(18) distance of 1.935 Å, indicating that the crystal has not reached the steady state yet (Figure S41). On the other hand, (S,S)-4BDHP-2-Vis-14h has a structure similar to (S,S)-4BDHP-2-Vis-7h, where the C(17)⋅⋅⋅C(18) distance is 2.231 Å, implying that the isomerization was once saturated (Figure S42). Further irradiation resulted in a yellow-colored crystal, which showed significantly weak diffraction intensity due to loss of crystallinity. The photo-isomerization, however, strongly depends on size and shape of single crystals due to relatively low quantum yield of the photo-isomerization reaction. Therefore, even single crystals are subjected to the same irradiation time, crystallographic analysis of the resultant crystals shows different degree of photo-isomerization depending on the crystals, preventing quantitative analysis for bulk crystals.
Finally, the thermal ring-closing reaction that occurred in a crystalline state was investigated. Heating of a photoisomerized crystal of (S,S)-4BDHP-2 resulted in color changes of the crystal from brown to dark brown (Figure S43). Crystallographic analysis of the heated crystal showed decrease of the C(3a1)−C(5a1) distance (Figure S44, Table S11). These results indicate the isomerization reaction from 4CPD to 4BDHP also occurred.
Conclusion
This study demonstrated a proof-of-concept system of photochromic H-bonded crystalline frameworks that underwent photo-isomerization while preserving crystalline periodicity and single crystallinity. The system was established by incorporating dihydrodimethylbenzo[e]pyrene (BDHP) as a photochromic moiety into the framework. The di- and tetracarboxylic acid derivatives 2BDHP and 4BDHP were synthesized, and their photo-isomerization behaviors were investigated in solution and crystalline states. Particularly, 4BDHP yielded H-bonded crystalline frameworks 4BDHP-1 and 4BDHP-2, the latter of which underwent photo-isomerization in the crystalline state because of the more loosely packed structure. Furthermore, its enantio-pure crystal (S,S)-4BDHP-2 allowed to reveal the crystal structure after photo-isomerization. In the photoisomerized crystalline framework, the closed form (4BDHP) and open form (4CPD) are arranged alternately while maintaining periodicity of the network. The present work can contribute to the development of new light-driven porous functional materials.
Supporting Information
The authors have cited additional references within the Supporting Information.57-63 Synthetic procedure, experimental details, theoretical calculations, detailed crystal structures, thermal analyses, and NMR spectra of the synthesized compounds (PDF), and crystal structures of BDHP (CIF), 2BDHP-1 (CIF), 2BDHP-X (CIF), 4BDHP-1 (CIF), 4BDHP-1-Vis (CIF), 4BDHP-2 (CIF), 4BDHP-2-Vis (CIF), (S,S)-4BDHP-1 (CIF), (S,S)-4BDHP-2 (CIF), (S,S)-4BDHP-2-2 (CIF), (S,S)-4BDHP-2-3 (CIF), (S,S)-4BDHP-2-4 (CIF), (S,S)-4BDHP-2-5 (CIF), (S,S)-4BDHP-2-Vis-4h (CIF), (S,S)-4BDHP-2-Vis-7h (CIF), (S,S)-4BDHP-2-Vis-7h-D (CIF), and (S,S)-4BDHP-2-Vis-14h (CIF). CCDC 2310094–2310106, 2310931, and 2345508 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing [email protected], or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Acknowledgments
This work was supported by KAKENHI (JP21K18961, JP21H01919, JP21H05485, JP22H05461, JP23H04029, and JP24 K01468) from JSPS and MEXT Japan. I. H. thanks Tokuyama Science Foundation, Hoansha Foundation, and Iketani Science and Technology Foundation for their financial support. I. H. thanks the Multidisciplinary Research Laboratory System for Future Developments (MRL), Graduate School of Engi-neering Science, Osaka University. The authors thank the Cybermedia Center, Osaka University, for use of the Super-computer for Quest to Unsolved Interdisciplinary Datascience (SQUID). The authors acknowledge Prof. Dr. N. Tohnai for X-ray diffraction analysis using Cu-radiation and Ms. R. Miyake for HR-MS analysis. Synchrotron X-ray diffraction data were collected at BL40XU at SPring-8 with approval of the Japan Synchrotron Radiation Research Institute (JASRI, proposal Nos. 2022A1093, 2022B1151, 2023B1142). Drs. K. Ichiyanagi and T. Sasaki are acknowledged for synchrotron radiation experiments.
Conflict of interests
The authors declare no conflict of interest.