A Simple and Sequential Strategy for the Introduction of Complexity and Hierarchy in Hydrogen-Bonded Organic Framework (HOF) Crystals for Environmental Applications
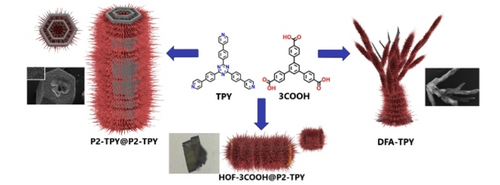
Graphical Abstract
Abstract
Hydrogen-bonded organic frameworks (HOFs) are a new class of crystalline porous organic molecular materials (POMMs) with great potential for a diverse range of applications. HOFs face common challenges to POMMs, and in general to purely organic crystals, that is, the difficulty of integrating complexity in crystals. Herein, we propose a simple and sequential strategy for the formation of HOFs with hierarchical superstructures. The strategy is based on controlling the assembly conditions, avoiding the use of any surface functionalization or template, which allows to obtain hierarchical crystalline porous superstructures in an easy manner. As proof of concept, we obtained the first example of core–shell (HOF-on-HOF) crystals and HOFs with hierarchical superstructures having superhydrophobicity and trapping abilities for the capture of persistent water contaminants such as oils and microplastics. We expect that this strategy could serve as inspiration for the construction of more intricate multiscale structures that could greatly expand the library of HOF materials.
Introduction
Porous organic molecular materials (POMMs) are a new class of synthetic materials characterized by the formation of porous frameworks mainly held together by non-covalent interactions.1 One type of POMMs are hydrogen-bonded organic frameworks (HOFs), forming porous frameworks held by hydrogen bonds and π–π stacking interactions during crystallization.2 HOFs have a unique combination of properties such as high crystallinity, high flexibility, lower weight, less inherent toxicity, recyclability, solution processability and self-healing properties. Since these properties are complementary to those with covalent and coordinated frameworks such as covalent organic frameworks (COFs)3 and metal–organic frameworks (MOFs),4 HOFs are considered excellent candidates for a vast range of applications.5 A clear evolution in the field of HOFs would be the integration of higher levels of ordered complexity6, 7 or hierarchy,8-10 during crystallization. Conceptually, by combining these hierarchies in a single material, novel materials with synergistic properties could be obtained, while expanding the number of HOF materials available in the toolbox. However, obtaining hierarchical crystals in purely organic materials11-14 such as HOFs is quite challenging due to the difficulty of controlling the weak interactions during crystallization, the different chemical stabilities between materials15 and strict lattice-matching requirements.16 Herein, we describe a template-free and versatile strategy, based on well controlled sequential crystallization steps from two molecular precursors, to obtain hierarchical HOF superstructures.17, 18 The simple assembly process allows us to obtain the first examples of purely organic HOF superstructures (Figure 1), showing unique features such as hierarchically branched nanostructures and core–shell (HOF-on-HOF) architecture. We based our strategy on the affinity of pyridine and carboxylic acid for the formation of strong hydrogen bonds and the compatibility in solubilities and stabilities between HOF precursors. With this simple strategy, commonly used approaches such as surface functionalization or the use of a template17, 19, 20 are not required. The versatile strategy opens the door for the design of HOF-based superstructures with unprecedented complexity.
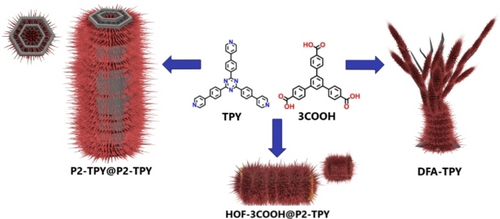
Schematic representation of the crystalline hierarchical superstructures obtained from two molecular precursors.
Results and Discussion
Crystalline Hierarchical Superstructures, DFA-TPY and P2-TPY@P2-TPY
The preparation of DFA-TPY (DFA-TPY: Double Fractal Architecture-Tripyridine) and P2-TPY@P2-TPY (P2-TPY: micro-macro Porosity-Tripyridine) starts from precursor TPY (Figure 2, Figure S1). The choice of solvent, concentration, and control of the evaporation rate at different stages is fundamental for the obtention of fractal superstructures. Initially, crystalline assemblies of FA-TPY (FA-TPY: Fractal Architecture-Tripyridine) with millimeter size and fractal-like morphology were obtained from a solution in toluene of TPY precursor (>1.2 mg/mL) at low solvent evaporation rate (>3 days). Then, FA-TPY was immersed in a solution of TPY in toluene (>0.8 mg/mL) and the solvent was left to evaporate at a fast rate (~20 hours) to obtain the final assembly, DFA-TPY. The relatively fast nucleation of the smaller crystals, generated on the surface of the main FA-TPY crystalline assembly, yields a dense array of crystals. Scanning electron microscopy (SEM) confirmed the multiscale morphology, with a secondary generation of crystals growing at the surface of the larger FA-TPY assemblies, having average diameters of less than 1 micrometer (Figure 2c). During the crystallization process and after thermal activation, the crystalline integrity of DFA-TPY is maintained, as confirmed by powder X-ray diffraction (PXRD) (Figure S4) and matches with the phase of a recently reported microporous HOF.21 The thermal stability of the DFA-TPY building block was also studied by thermogravimetric analysis (TGA) under N2 (Figure S5). An initial and gradual 5 % weight loss below 300 °C was attributed to the loss of toluene within the packing. A second weight loss of >70 % between 400 °C and 500 °C was attributed to the thermal decomposition of TPY. This confirms that the DFA-TPY precursor is thermally stable below 400 °C.
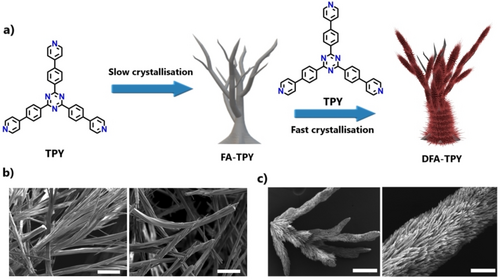
a) Schematic representation of the sequential crystallization of FA-TPY and DFA-TPY. b-c) SEM images of FA-TPY (b) and DFA-TPY (c). Scale bars (from left to right): (b) 100 μm, 100 μm, (c) 100 μm, 10 μm.
P2-TPY hollow crystals, used for the obtention of P2-TPY@P2-TPY (Figure 3), were also prepared from precursor TPY but at low concentration (<0.6 mg/mL) and low evaporation rate of toluene (>4 days). SEM images of the crystals revealed macropores within the main hollow interior (Figure 3b). The crystal structure of P2-TPY was solved by SCXRD analysis (Table S1). In the crystal packing, there are six TPY molecules and nine toluene molecules in the asymmetric unit. The packing is represented by the TPY molecules arranged in pairs and all six unique molecules forming an infinite π⋅⋅⋅π stacked column parallel to b (Figure 3d). The first molecule is adjacent to a symmetry equivalent of the sixth molecule. The closest contacts between atoms of central rings are significantly shorter than usual π⋅⋅⋅π stacking distances, between 3.26–3.35 Å. The spread of the centroid-centroid distances and pattern of closest contacts supports the monoclinic space group selection due to the pattern of variation. If the TPY molecules are regarded as being arranged in layers in the a/c plane, then the toluene molecules are in the formed cavity between every other layer in the spaces between stacks of molecules. There is no additional void volume or disorder. With the toluene molecules removed, there are three unique ‘voids’, so six in total in the unit cell. Each equates to about 5 % of the cell volume. The void volumes for each individual void are: 538, 545, and 530 Å3. Overall ca. 30 % of the cell volume is occupied by toluene molecules, thus defining the presence of micropores where the longest diagonal distance within each pore is close to 1.6 nm. Next, crystals of P2-TPY with micrometer size (Figure 3a), initially having hierarchical micro and macroporosity, were immersed in a solution of TPY precursor in toluene (<0.6 mg/mL) and the solvent was left to evaporate at a fast rate (~20 minutes). The relatively fast nucleation of the newly generated smaller crystals of P2-TPY on the surface of the main hollow P2-TPY crystals yields a dense array of nanocrystals with average diameters of 100–200 nm, covering most of the surface of the main hollow crystal, including the inner main hollow structure, thus forming superstructures with hierarchical architecture and porosity. SEM confirmed that the secondary generation of P2-TPY nanocrystals growing at the surface of larger crystals have rod morphology (Figure 3b) forming a multiscale morphology that resembles a nanoforest. This strategy is also versatile in terms of scale. For example, by modifying the size of the seed crystals of P2-TPY, from micrometer to millimeter size, a secondary generation of micrometer size crystals can be grown, which can be easily visualized by optical microscopy (Figure S6). During the crystallization process for the formation of P2-TPY@P2-TPY with hierarchical porosity and hierarchical architecture, the crystalline integrity of P2-TPY is maintained, as confirmed by powder X-ray diffraction (PXRD) (Figure S7). However, during the activation of P2-TPY@P2-TPY at 110 °C, single-crystal to single-crystal transformation occurred to form a new crystalline microporous phase, matching with the DFA-TPY phase (Figure 3d). The thermal stability of P2-TPY@P2-TPY was studied by TGA, confirming similar stability to DFA-TPY (Figure S8).
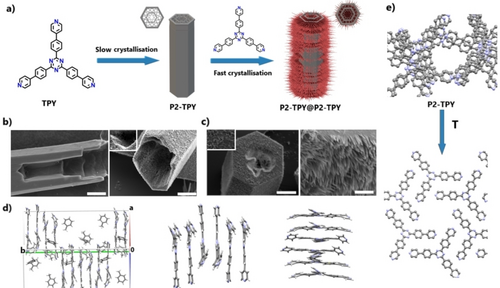
a) Schematic representation of the sequential crystallization of P2-TPY@P2-TPY. b–c) SEM images of P2-TPY (b Right) and P2-TPY@P2-TPY ((b Left), c). d) Crystal packing obtained from single crystal X-ray of P2-TPY, with different views of the molecular interactions in the packing arrangement showing the strong, slightly slipped π–π stacking. Blue: N, Grey: C. e) Changes in the crystal packing of P2-TPY (single-crystal to single-crystal transformation) to the same phase as DFA-TPY, previously reported.21 Scale bars (from left to right): (b) 10 μm, (c) 10 μm (Left), 1 μm (Right). Hydrogens have been omitted for clarity.
Crystalline Core-Shell Superstructures (HOF-on-HOF), HOF-3COOH@P2-TPY
First, crystals of HOF-3COOH were obtained from slow crystallization (~2 weeks) of a methanol solution of 3COOH (~1.4 mg/mL) precursor at room temperature (Figure 4). SCXRD was used to determine the crystal packing of HOF-3COOH (Table S2). Packing in the unit cell is accommodated by both ring π–π stacking and the interlocking together of the 3COOH molecules, dominated by hydrogen bonding involving all three carboxyl COOH groups (Figure 4b). The rotatable bond of the carboxylic acid allows for two possible hydrogen bonding orientations in the crystal lattice seen here, generating an average bond length for OH carboxyl donor atoms. The 3COOH molecules interact through π⋅⋅⋅π stacking of the central aromatic ring as a column consisting of sets four molecules plus three molecules, packed as a herringbone arrangement resulting in twenty-eight (4×7) independent molecules in the ASU. The total solvent-accessible volume is 29404 Å3 made from four equivalent ‘voids’ of 7351 Å3 or 8.7 % each. The overall void volume is 14750 Å3 or 17 % of the unit cell volume (84656 Å3), when calculated using solvent molecule of diameter of 1.4 Å. There is a continuous single channel running along the a/c axes22 (Figure S2) with the narrowest region accommodating a probe molecule radius of 2 Å3 with the other two dimensions of about 1.4×1.6 nm. Within the channels there is no fixed ordering of solvent based on the residual electron density maps. The thermal stability of HOF-3COOH building block measured by the TGA curve under N2 (Figure S9) experienced an initial 5 % weight loss below 80 °C, attributed to the loss of solvent within the pores. A second weight loss of >20 % between 200 °C and 600 °C was attributed to any remaining solvent trapped and the thermal decomposition of the molecular precursors. The CO2 adsorption isotherm at 223 K of HOF-3COOH shows an uptake capacity of 3.28 mmol/g (Figure S10), which can be considered excellent for a HOF.5, 24 Next, HOF-3COOH crystals were immersed in a warm solution of TPY in toluene (0.6 mg/mL) and the solvent was left to evaporate at a slow rate (~18 hours), yielding HOF-on-HOF crystals of HOF-3COOH@P2-TPY. SEM confirmed the core–shell morphology of P2-TPY as nanometric crystalline rods (Figure 4d) growing on the surface of HOF-3COOH. EDS analysis of HOF-3COOH@P2-TPY for C, N and O elements showed the presence of C and N at the shell and only C and O at the solid core of the crystal (Figure 4e–f, Figure S11). PXRD also confirmed the presence of the P2-TPY phase and the maintained integrity of HOF-3COOH phase, (Figure S12–S13). FTIR analysis of HOF-3COOH@P2-TPY crystals confirmed the presence of characteristic peaks of P2-TPY and HOF-3COOH (Figure S14). The dissimilar parameters for the unit cells in both crystals (a=54.6 Å, b=31.2 Å, c=54.5 Å, β=114.6 Å vs a=17.4 Å, b=40.0 Å, c=17.4 Å, β=119.9 Å) suggest a lattice mismatch. To shed light on the driving force for the core–shell formation, and the possible types of interactions at the interface, we co-crystallized 3COOH and TPY obtaining crystals (Hybrid-1) that were characterized by SCXRD (Table S3). The crystals of Hybrid-1 revealed an asymmetric unit consisting of seven pairs of 3COOH molecules forming hydrogen bonds to TPY in a 1 : 1 ratio (more detailed description of the crystal packing in the SI). H-bonding interactions are observed between all three arms of TPY and 3COOH through the N pyridyl acceptor and OH carboxyl donor atoms (2.49 Å average) (Figure S3). The presence of H-bonding interactions is not surprising, as numerous studies have demonstrated the stability of H-bonds between pyridine and carboxylic groups.23 Further stabilizing interactions consisted of π–π stacking interactions between the central triazine ring, central phenyl rings and outer pyridyl rings with a step of around 3.34 Å between the planes.
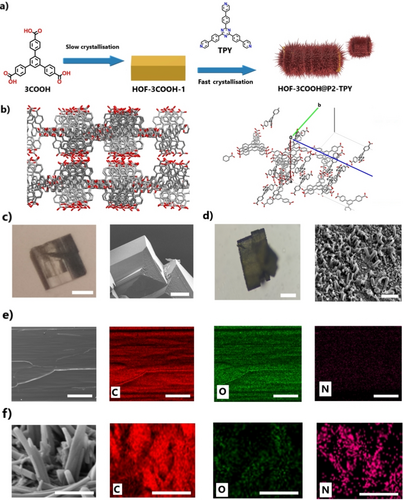
a) Schematic representation of the sequential crystallization of HOF-3COOH@P2-TPY. b) Different views of the crystal packing of HOF-3COOH showing the channels and the molecular interactions in the packing arrangement with the strong COOH-COOH H-bonds and π–π stacking and the asymmetric unit. Red: O, Grey: C. All hydrogens have been omitted for clarity. c-d) Optical (Left) and SEM (Right) images of HOF-3COOH (c) and HOF-3COOH@P2-TPY (d). e–f) EDS mapping of C, O and N for HOF-3COOH (e) and 3COOH@P2-TPY (f Scale bars (from left to right): (c) 100 μm, (d) 50 μm, 1 μm, (e) 10 μm, (f) 1 μm.
Based on the crystal data obtained for Hybrid-1, HOF-3COOH and P2-TPY, we calculated the energy of H-bonding interactions between pairs. DFT calculations were performed using version 5.0.3 of the ORCA program.25 The range-separated hybrid functional, ωB97X, was used to approximate the exchange–correlation functional. The polarized triple-zeta basis set, def2-TZVP,26 as well as the general auxiliary basis, Def2/J,27 were used in the calculations. The values in energy for both contributions, H-bonding and π–π stacking, account for 12 kcal/mol and 27 kcal/mol, respectively. The H-bond interaction calculation was repeated using the M06-2X functional so that this result can be directly compared with previous work.21 The obtained energy value is very similar at 11.1 kcal/mol for the H-bond interaction on the co-crystals of Hybrid-1 for COOH….N. This is significantly higher than the H-bond value of 5.4 kcal/mol for N….N in P2-TPY seen in previous works.21 This suggests that the driven mechanism of growth of P2-TPY on the surface of HOF-3COOH would be the stronger H-bond interactions that originated at the interface between TPY and 3COOH crystals. The thermal stability of HOF-3COOH@P2-TPY building blocks was also studied by TGA under N2. The TGA curve (Figure S17) experienced an initial 8 % weight loss below 100 °C, attributed to the loss of solvents within the pores. A second weight loss of >50 % between 200 °C and 450 °C was attributed to any remaining solvent trapped within the pores and the thermal decomposition of the molecular precursors. The porosity of HOF-3COOH@P2-TPY was also studied by gas adsorption after sample activation (Figure S18). The CO2 adsorption isotherm at 223 K of HOF-3COOH@P2-TPY shows an uptake capacity of 2.3 mmol/g, which it is expected, corresponding to an intermediate adsorption between both HOFs. To our knowledge, this is the first stable HOF-on-HOF crystal and the first POMM using dissimilar building blocks.16
Superhydrophobicity, Hierarchy, and Contaminant Trapping
Hierarchy in HOFs could be used as an elegant route for the introduction of multifunctionality in crystalline materials for specific applications. For example, the multiscale nature of some of these materials could be used to introduce special wettability and trapping properties. Previous studies have demonstrated that hierarchical materials can alter the surface's wettability.28 However, the wettability of HOFs has been hardly explored, with only a few reports published, showing the amphiphilic nature of building blocks due to the presence of aromatic hydrophobic blocks and hydrophilic functional groups.29-31 To increase the hydrophobic nature of HOFs, previous reports were based on designing fully fluorinated pores.29 We propose that multiscale HOFs could be good candidates as hydrophobic materials. Some of these hierarchical materials display low wettability in the presence of water, forming liquid marbles and floating on water (Figure S19a). In fact, when we measured the contact angle for P2-TPY@P2-TPY crystals, angles of >150° were obtained, which can be considered as superhydrophobic (Figure 5a). To our knowledge, this is the first reported HOF with superhydrophobic properties. The high hydrophobicity was also demonstrated for DFA-TPY (Figure 5b) and HOF-3COOH@P2-TPY (Figure S19b). The hydrophobicity and hierarchical fractal nature of DFA-TPY could be advantageous in applications for challenging separations such as selective oil-water adsorption and microplastics trapping, as they are two of the most relevant environmental contaminants. Oil in water is a well-known and persistent contaminant32 and, similarly, microplastics can be found in the environment and even the human body, with the toxicity that entails.33 Traditional methods for the removal of microplastics in wastewater plants are not effective34 and new methods are required.35, 36
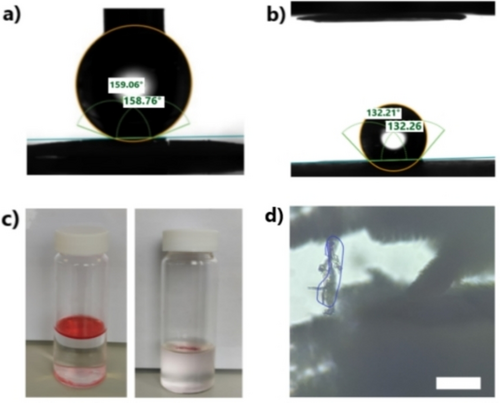
a–b) Contact angle measurements for P2-TPY@ P2-TPY (a) and DFA-TPY (b). c) Images of oil in water dyed with Oil Red before (left) and after adding DFA-TPY (right). d) Optical microscopy image of DFA-TPY with trapped microplastic after filtration. Scale bar: 10 μm.
As a proof of concept, we tested DFA-TPY for the removal of both oil and solid contaminants from water. When a mixture of water and hexane or petroleum ether (Figure 5c) dyed with Oil Red were prepared, DFA-TPY was able to completely remove 99 % of the oil in minutes, proving the efficacy of hydrophobicity, with no visual evidence of remaining oil. For microplastics, a water solution containing PET microplastics was filtered through DFA-TPY, with 98 % of the total amount of microplastics removed. These efficacies for the capture of oil and microplastics are comparable with the state-of-the-art materials for both applications.37, 38 The mechanism of microplastic trapping is expected to be favored by hydrophobic interactions and the hierarchical nature of the fractal DFA-TPY (Figure 5d). Beyond its efficiency in removing contaminants and due to its crystalline nature, DFA-TPY can be easily regenerated and after the end of its life, recrystallized and reused again.
Conclusion
The ability to prepare crystalline organic porous materials with hierarchical superstructures is a current challenge in the field of POMMs, and particularly in HOFs. Herein, we have introduced the first examples of organic superstructures based on HOFs by using a controlled and sequential crystallization strategy, without the need of using templates or surface functionalization. We also demonstrated the potential of these superstructures to confer superhydrophobicity and trapping abilities for the capture of persistent water contaminants such as oils and microplastics. This work deepens our understanding of multiscale hierarchies, opening an avenue to design multifunctional HOFs such as HOF-on-HOF structures, where this strategy could serve of inspiration for the construction of more intricate multiscale and heterogeneous porous organic materials with optimized properties, as well as greatly expanding the library of HOF materials.
Supporting Information
The authors have cited additional references within the Supporting Information.21, 39-41
Acknowledgments
A.F. thanks to the Royal Society (RGS\R1\221390) for the funding. We also thank the UK. EPSRC, National Crystallography Service at the University of Southampton for collection of single crystal X-ray data.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Supplementary crystallographic data for this paper are provided free of charge by the joint Cambridge Crystallographic Data Centre.42