The Tandem Nitrate and CO2 Reduction for Urea Electrosynthesis: Role of Surface N-Intermediates in CO2 Capture and Activation
Xingmiao Huang
Key Laboratory of Photochemistry, Institute of Chemistry Chinese Academy of Sciences, Beijing National Laboratory for Molecular Sciences, 100190 Beijing, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorYangfan Li
Key Laboratory of Photochemistry, Institute of Chemistry Chinese Academy of Sciences, Beijing National Laboratory for Molecular Sciences, 100190 Beijing, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorCorresponding Author
Dr. Shijie Xie
State Key Laboratory of Fine Chemical, Frontiers Science Center for Smart Materials Oriented Chemical Engineering, School of Chemical Engineering, Dalian University of Technology, 116024 Dalian, P. R. China
Search for more papers by this authorQi Zhao
Key Laboratory of Photochemistry, Institute of Chemistry Chinese Academy of Sciences, Beijing National Laboratory for Molecular Sciences, 100190 Beijing, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorBoyang Zhang
Key Laboratory of Photochemistry, Institute of Chemistry Chinese Academy of Sciences, Beijing National Laboratory for Molecular Sciences, 100190 Beijing, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorZhiyong Zhang
Key Laboratory of Photochemistry, Institute of Chemistry Chinese Academy of Sciences, Beijing National Laboratory for Molecular Sciences, 100190 Beijing, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Hua Sheng
Key Laboratory of Photochemistry, Institute of Chemistry Chinese Academy of Sciences, Beijing National Laboratory for Molecular Sciences, 100190 Beijing, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorProf. Jincai Zhao
Key Laboratory of Photochemistry, Institute of Chemistry Chinese Academy of Sciences, Beijing National Laboratory for Molecular Sciences, 100190 Beijing, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorXingmiao Huang
Key Laboratory of Photochemistry, Institute of Chemistry Chinese Academy of Sciences, Beijing National Laboratory for Molecular Sciences, 100190 Beijing, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorYangfan Li
Key Laboratory of Photochemistry, Institute of Chemistry Chinese Academy of Sciences, Beijing National Laboratory for Molecular Sciences, 100190 Beijing, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorCorresponding Author
Dr. Shijie Xie
State Key Laboratory of Fine Chemical, Frontiers Science Center for Smart Materials Oriented Chemical Engineering, School of Chemical Engineering, Dalian University of Technology, 116024 Dalian, P. R. China
Search for more papers by this authorQi Zhao
Key Laboratory of Photochemistry, Institute of Chemistry Chinese Academy of Sciences, Beijing National Laboratory for Molecular Sciences, 100190 Beijing, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorBoyang Zhang
Key Laboratory of Photochemistry, Institute of Chemistry Chinese Academy of Sciences, Beijing National Laboratory for Molecular Sciences, 100190 Beijing, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorZhiyong Zhang
Key Laboratory of Photochemistry, Institute of Chemistry Chinese Academy of Sciences, Beijing National Laboratory for Molecular Sciences, 100190 Beijing, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Hua Sheng
Key Laboratory of Photochemistry, Institute of Chemistry Chinese Academy of Sciences, Beijing National Laboratory for Molecular Sciences, 100190 Beijing, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorProf. Jincai Zhao
Key Laboratory of Photochemistry, Institute of Chemistry Chinese Academy of Sciences, Beijing National Laboratory for Molecular Sciences, 100190 Beijing, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorGraphical Abstract
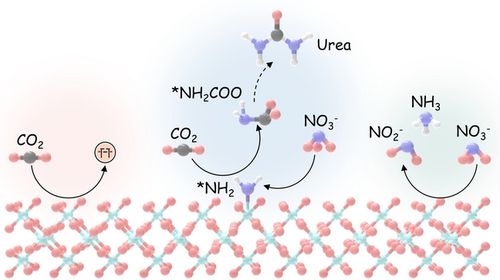
The electrocatalytic coupling reaction of carbon dioxide and nitrate serves as a sustainable strategy for urea synthesis under ambient conditions. Herein we proposed a urea generation pathway via the tandem reduction of nitrate and CO2, in which the critical C−N coupling is achieved by the interaction between the nitrate reduction intermediate of *NH2 and free CO2.
Abstract
Electrochemical reduction of CO2 and nitrate offers a promising avenue to produce valuable chemicals through the using of greenhouse gas and nitrogen-containing wastewater. However, the generally proposed reaction pathway of concurrent CO2 and nitrate reduction for urea synthesis requires the catalysts to be both efficient in both CO2 and nitrate reduction, thus narrowing the selection range of suitable catalysts. Herein, we demonstrate a distinct mechanism in urea synthesis, a tandem NO3− and CO2 reduction, in which the surface amino species generated by nitrate reduction play the role to capture free CO2 and subsequent initiate its activation. When using the TiO2 electrocatalyst derived from MIL-125-NH2, it intrinsically exhibits low activity in aqueous CO2 reduction, however, in the presence of both nitrate and CO2, this catalyst achieves an excellent urea yield rate of 43.37 mmol ⋅ g−1 ⋅ h−1 and a Faradaic efficiency of 48.88 % at −0.9 V vs. RHE in a flow cell. Even at a low CO2 level of 15 %, the Faradaic efficiency of urea synthesis remains robust at 42.33 %. The tandem reduction procedure was further confirmed by in situ spectroscopies and theoretical calculations. This research provides new insights into the selection and design of electrocatalysts for urea synthesis.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202403980-sup-0001-misc_information.pdf2.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Lim, C. A. Fernández, S. W. Lee, M. C. Hatzell, ACS Energy Lett. 2021, 6, 3676–3685.
- 2
- 2aX. Zhang, E. A. Davidson, D. L. mauzerall, T. D. searchinger, P. Dumas, Y. shen, Nature 2015, 528, 51–59;
- 2bJ. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont, W. Winiwarter, Nat. Geosci. 2008, 1, 636–639;
- 2cP. M. Glibert, J. Harrison, C. Heil, S. Sybil, Biogeochemistry 2006, 77, 441–463.
- 3
- 3aM. Aresta, A. Dibenedetto, A. Angelini, Chem. Rev. 2013, 114, 1709–1742;
- 3bJ. Qiao, Y. Liu, F. Hong, J. Zhang, Chem. Soc. Rev. 2014, 43, 631–675.
- 4F. Barzagli, F. Mania, M. Peruzzinib, Green Chem. 2011, 13, 1267–1274.
- 5D. R. MacFarlane, P. V. Cherepanov, J. Choi, B. H. R. Suryanto, R. Y. Hodgetts, J. M. Bakker, F. M. Ferrero Vallana, A. N. Simonov, Joule 2020, 4, 1186–1205.
- 6
- 6aC. Tang, Y. Zheng, M. Jaroniec, S. Qiao, Angew. Chem. Int. Ed. 2021, 60, 19572–19590;
- 6bZ. Tao, C. L. Rooney, Y. Liang, H. Wang, J. Am. Chem. Soc. 2021, 143, 19630–19642;
- 6cJ. Li, Y. Zhang, K. Kuruvinashetti, N. Kornienko, Nat. Chem. Rev. 2022, 6, 303–319.
- 7
- 7aM. Shibata, K. Yoshida, N. Furuya, J. Electroanal. Chem. 1998, 442, 67–72 ;
- 7bM. Shibata, K. Yoshida, N. Furuya, J. Electrochem. Soc. 1998, 145, 595–600;
- 7cM. Shibata, N. Furuya, J. Electroanal. Chem. 2001, 507, 177–184;
- 7dM. Shibata, K. Yoshida, N. Furuya, Denki Kagaku 1998, 66, 584–589.
- 8
- 8aY. Luo, K. Xie, P. Ou, C. Lavallais, T. Peng, Z. Chen, Z. Zhang, N. Wang, X. Y. Li, I. Grigioni, B. Liu, D. Sinton, J. B. Dunn, E. H. Sargent, Nat. Catal. 2023, 6, 939–948;
- 8bM. Jiang, M. Zhu, M. Wang, Y. He, X. Luo, C. Wu, L. Zhang, Z. Jin, ACS Nano 2023, 17, 3209–3224.
- 9J. Liu, X. Guo, T. Frauenheim, Y. Gu, L. Kou, Adv. Funct 2024, 2313420.
- 10
- 10aJ. Liu, S. C. Smith, Y. Gu, L. Kou, Adv. Funct 2023, 202305894;
- 10bJ. Liu, X. Lv, Y. Ma, S. C. Smith, Y. Gu, L. Kou, ACS Nano 2023, 17, 25667–25678.
- 11
- 11aX. Zhang, X. Zhu, S. Bo, C. Chen, M. Qiu, X. Wei, N. He, C. Xie, J. Zheng, P. Chen, S. Jiang, Q. Liu, S. Wang, Nat. Commun. 2022, 13, 5337–5345;
- 11bY. Zhao, Y. Ding, W. Li, C. Liu, Y. Li, Z. Zhao, Y. Shan, F. Li, L. Sun, F. Li, Nat. Commun. 2023, 14, 4491.
- 12
- 12aY. Mao, Y. Jiang, Q. Gou, S. Lv, Z. Song, Y. Jiang, W. Wang, M. Li, L. Zheng, W. Su, R. He, Appl. Catal. B 2024, 340, 123189–123199;
- 12bP. Lu, J. Wang, F. Lu, Q. Liu, Y. Gao, Y. Wang, J. Jiang, C. Sun, J. Wang, X. Wang, Angew. Chem. Int. Ed. 2023, 62, e202216835;
- 12cJ. Geng, S. Ji, M. Jin, C. Zhang, M. Xu, G. Wang, C. Liang, H. Zhang, Angew. Chem. Int. Ed. 2022, 62, e202210958.
- 13
- 13aY. Wang, W. Zhou, R. Jia, Y. Yu, B. Zhang, Angew. Chem. Int. Ed. 2020, 59, 5350–5354;
- 13bZ. Song, Y. Liu, Y. Zhong, Q. Guo, J. Zeng, Z. Geng, Adv. Mater. 2022, 34, e2204306.
- 14
- 14aH. Yamada, J. Phys. Chem. B 2016, 120, 10563–10568;
- 14bH. Yamada, S. Shimizu, H. Okabe, Y. Matsuzaki, F. A. Chowdhury, Y. Fujioka, Ind. Eng. Chem. Res. 2012, 49, 2449–2455.
- 15C. Lv, L. Zhong, H. Liu, Z. Fang, C. Yan, M. Chen, Y. Kong, C. Lee, D. Liu, S. Li, J. Liu, L. Song, G. Chen, Q. Yan, G. Yu, Nat. Sustain. 2021, 4, 868–876.
- 16G. Yang, C. Hsieh, Y. Ho, T. Kuo, Y. Kwon, Q. Lu, M. Cheng, ACS Catal. 2022, 12, 11494–11504.
- 17
- 17aC. Chen, X. Zhu, X. Wen, Y. Zhou, L. Zhou, H. Li, L. Tao, Q. Li, S. Du, T. Liu, D. Yan, C. Xie, Y. Zou, Y. Wang, R. Chen, J. Huo, Y. Li , J. Cheng, H. Su, X. Zhao, W. Cheng, Q. Liu , H. Lin, J. Luo, J. Chen , M. Dong , K. Cheng, C. Li , S. Wang, Nat. Chem. 2020, 12, 717–724;
- 17bD. Saravanakumar, J. Song, S. Lee, N. H. Hur, W. Shin, ChemSusChem 2017, 10, 3999–4003.
- 18U. Balachandran, N. Eror, J. Solid State Chem. 1982, 42, 276–282.
- 19
- 19aB. Yan, D. Liu, X. Feng, M. Shao, Y. Zhang, Adv. Funct. Mater. 2020, 30, 2003007;
- 19bY. Yang, J. Zhu, P. Wang, H. Liu, W. Zeng, L. Chen, Z. Chen, S. Mu, Acta Phys. -Chim. Sin. 2022, 38, 2106002.
- 20T. Wu, H. Zhao, X. Zhu, Z. Xing, Q. Liu, T. Liu, S. Gao, S. Lu, G. Chen, A. M. Asiri, Y. Zhang, X. Sun, Adv. Mater. 2020, 32, 202000299.
- 21Q. Qin, Y. Zhao, M. Schmallegger, T. Heil, J. Schmidt, R. Walczak, G. Gescheidt-Demner, H. Jiao, M. Oschatz, Angew. Chem. Int. Ed. 2019, 58, 13101–13106.
- 22Z. Han, C. Choi, S. Hong, T. Wu, Y. Soo, Y. Jung, J. Qiu, Z. Sun, Appl. Catal. B 2019, 257, 117896–117904.
- 23
- 23aM. Shibata, Y. Kohji, N. Furuya, J. Electroanal. Chem. 1995, 387, 143–145;
- 23bX. Zhang, X. Zhu, S. Bo, C. Chen, K. Cheng, J. Zheng, S. Li, X. Tu, W. Chen, C. Xie, X. Wei, D. Wang, Y. Liu, P. Chen, S. P. Jiang, Y. Li, Q. Liu, C. Li, S. Wang, Angew. Chem. Int. Ed. 2023, e202305447.
- 24
- 24aY. Li, E. P. Delmo, G. Hou, X. Cui, M. Zhao, Z. Tian, Y. Zhang, M. Shao, Angew. Chem. Int. Ed. 2023, e202313522;
- 24bW. Lyu, Y. Liu, J. Zhou, D. Chen, X. Zhao, R. Fang, F. Wang, Y. Li, Angew. Chem. Int. Ed. 2023, e202310733.
- 25M. Xu, F. Wu, Y. Zhang, Y. Yao, G. Zhu, X. Li, L. Chen, G. Jia, X. Wu, Y. Huang, P. Gao, W. Ye, Nat. Commun. 2023, 14, 6994–7005.
- 26Y. Yao, S. Zhu, H. Wang, H. Li, M. Shao, J. Am. Chem. Soc. 2018, 140, 1496–1501.
- 27S. Han, H. Li, T. Li, F. Chen, R. Yang, Y. Yu, B. Zhang, Nat. Catal. 2023, 6, 402–414.
- 28W. Wang, C. Deng, S. Xie, Y. Li, W. Zhang, H. Sheng, C. Chen, J. Zhao, J. Am. Chem. Soc. 2021, 143, 2984–2993.
- 29E. P. Delmo, Y. Wang, Y. Song, S. Zhu, H. Zhang, H. Xu, T. Li, J. Jang, Y. Kwon, Y. Wang, M. Shao, J. Am. Chem. Soc. 2024, 146, 1935–1945.
- 30X. Tu, X. Zhu, S. Bo, X. Zhang, R. Miao, G. Wen, C. Chen, J. Li, Y. Zhou, Q. Liu, D. Chen, H. Shao, D. Yan, Y. Li, J. Jia, S. Wang, Angew. Chem. Int. Ed. 2023, e202317087.
- 31J. Y. Fang, Q. Z. Zheng, Y. Y. Lou, K. M. Zhao, S. N. Hu, G. Li, O. Akdim, X. Y. Huang, S. G. Sun, Nat. Commun. 2022, 13, 7899–7911.
- 32K. M. Khan, H. Krishna, S. K. Majumder, P. K. Gupta, Food Anal. Methods 2014, 8, 93–102.
- 33
- 33aJ. Jehlička, H. G. M. Edwards, A. Culka, Phil. Trans. R. Soc. A 2010, 368, 3109–3125;
- 33bR. Keuleers, H. O. Desseyn, B. Rousseau, C. V. Alsenoy, J. Phys. Chem. A 1999, 103, 4621–4630.
- 34X. Chang, S. Vijay, Y. Zhao, N. J. Oliveira, K. Chan, B. Xu, Nat. Commun. 2022, 13, 2656–2667.