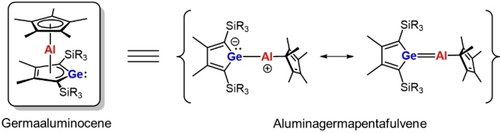
Germaaluminocenes—Masked Heterofulvenes
Graphical Abstract
The reactivity pattern of germaaluminocenes is determined by the dichotomy of the electron-deficient aluminum atom and the electron-rich germanium center. The analysis of the product spectra obtained from the reaction with standard reagents reveals the role of these main group sandwich complexes as synthetic equivalents of otherwise not accessible aluminagermapentafulvenes.
Abstract
Germaaluminocenes are formed by salt metathesis reactions of dipotassium germacyclopentadienediides with pentamethylcyclopentadienylaluminum dichloride. The reactivity pattern of these sandwich complexes is determined by the electrophilic central aluminum atom and by the nucleophilic dicoordinated germanium center. Surprisingly, the products formed by reactions with Lewis acids, Lewis bases, amphiphiles and compounds with polar double bonds are those expected from the reaction of a hypothetical aluminagermapentafulvene with these types of reagents. This suggests that germaaluminocenes are synthetic equivalents to these pentafulvenes.
Introduction
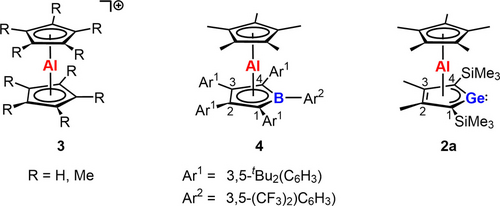
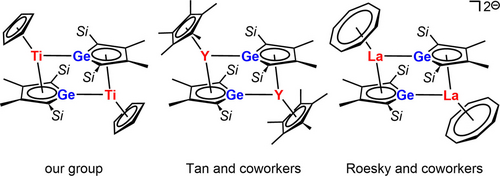
Heteroalkenes, compounds with double bonds between two different elements, are in the focus of modern molecular main group chemistry.1-3 The resulting polarization of both constituting bonds, the σ- and the π-bond, and the small energy gap between these bonding orbitals and their antibonding counterparts make such compounds interesting synthetic targets. The spatial and energetic arrangement of the frontier orbitals in heteroalkenes mimic the electronic situation in transition metal complexes, which are widely exploited in bond activation chemistry and, hence, in molecular catalysis. Heteroalkenes based on main group elements such as aluminum or silicon have the advantage of long-term availability and low toxicity compared to transition metals established in the field of bond activation. The activation of small molecules by heteroalkenes formed from one of these two elements has been demonstrated.3-9 We started our investigation in this field with the attempted synthesis of heterofulvenes 1.10 In these cases, the polarization of the double bond is enforced by the involvement of canonical structures with 6π-delocalized heterocyclopentadiene motifs like 1B, which should be beneficial for bond activation reactions (Figure 1). The enhanced polarization of the fulvenes 1 triggers however intramolecular rearrangements. For the combination E=Ge and E′R=AlCp* (Cp*: pentamethylcyclopentadienyl) the heterofulvene structure is not stable and bis-η5-sandwich compounds such as 2, are formed (Figure 1).11 Quite a number of sandwich complexes with tetrole perimeter have been prepared and their synthesis and reactivity has been recently summarized by Saito12 and Sun and Roesky.13 Besides the archetypical cationic aluminocenes 3,14-18 the only known aluminum based sandwich complexes are the neutral borole complexes 4 reported by Sindlinger and co-workers19 and the germanium derivative 2 a prepared in our group (Scheme 1).11 For related complexes of germole dianions with trivalent metal ions such as Ti3+, Y3+ and also La3+ and Ce3+ the formation of dimers is reported (Scheme 2),20-22 whereas the smaller lanthanoid ions (Er3+, Dy3+) form monomeric cyclooctatetraenediide germolediide sandwich complexes.23 The dimerization indicates the conserved Lewis basic reactivity of the germanium center and Lewis acidity of the metal ions. This interesting unquenched Lewis acid/base pattern, also present in the germaaluminocenes 2, calls for an extended reactivity study. Here we report on the synthesis of germaaluminocenes 2 including a derivative 2 b with bulkier flanking silyl substituents at the germole ring and on their fundamental reactivity pattern versus standard reagents which suggests that these sandwich compounds behave as synthetic equivalents to heterofulvenes 1.
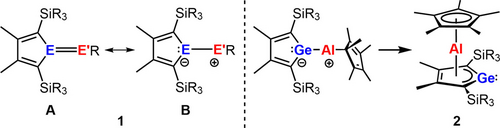
Heterofulvenes 1 (E=Si, Ge; E'R=BR, AlR; SiR3=SiMe3, SiMe2tBu) and formation of the sandwich complex 2.11
Results and Discussion
The double salt metathesis reaction of dipotassium germacyclopentadienediide K2[5 b] with the aluminum dichloride 6 provided germaaluminocene 2 b in isolated yields up to 77 % crystalline material (Scheme 3).24 The significant higher yield of the tBuMe2Si substituted germaaluminocene 2 b compared to 2 a is a result of its better crystallization ability. Germaaluminocene 2 b was characterized by multinuclear NMR spectroscopy, high resolution mass spectrometry (HRMS) and by single crystal X-ray diffraction (sc-XRD) analysis. As expected, the NMR spectroscopic results are similar to those reported previously for 2 a (see Supporting Information Material for details).11 Of significance is the relative sharp δ27Al signal (full width at half height: ω1/2=690 Hz) at the expected region for aluminocene compounds (δ27Al=−81.4 vs. δ27Al=−115 (ω1/2=8 Hz) (3, R=Me),14 δ27Al=−86.2 (ω1/2=2.6 kHz) (4)19 and δ27Al=−77.6 (ω1/2=703 Hz) (2 a)).11 The 13C NMR chemical shifts of the ring carbon atoms C1/4 and C2/3 indicate a delocalized bonding situation for the germanium heterocycle. Similar to the aromatic dianions [5]2− the carbon atoms C1/4 in α-position to the germanium atom are more deshielded than the carbon atoms C2/3 (δ13C(1/4)=164.1, (δ13C(2/3)=146.1, see Scheme 3 and Table S13).25
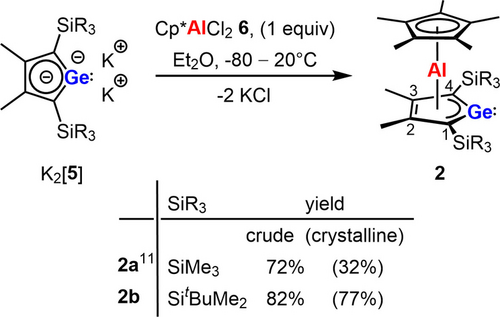
Synthesis of neutral germaaluminocenes 2.
Recrystallization from nhexane gave yellow crystals of aluminocene 2 b suitable for sc-XRD analysis (Figure 2). Its molecular structure in the crystal is very close to that of the cognate compound 2 a.11 Here we want to emphasize the short distances between the aluminum atom and the ring carbon atoms of the Cp* substituent and all atoms of the germole heterocycle indicating the bis-η5-coordination of the aluminum atom. Two noteworthy structural details are the short aluminum germanium distance (245.7 pm), which is shorter than the theoretically predicted Ge−Al single bond length of 247 pm,26 and the markedly smaller Al−C1/C4 distances compared to the Al−C2/3 separations (Al−C1/C4=214–215 pm vs. Al−C2/C3=230–231 pm, see Figure 2). The almost equal lengths of the C−C bonds in the germole ring (142–145 pm) indicate its high degree of delocalization, which is in qualitative agreement with the NMR experiments. The Ge−C1/4 and all C−C bonds of the heterocycle are longer than in the anions [5]2−,25 indicating the electron transfer from the germole ring to the aluminum atom (Table S14).
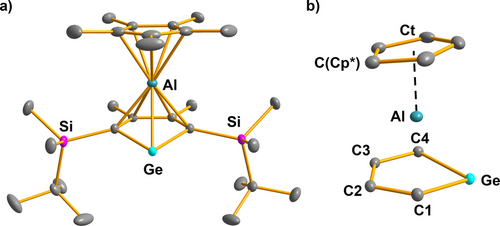
a) Molecular structure of germaaluminocene 2 b in the crystal (Hydrogen atoms are omitted, thermal ellipsoids with 50 % probability). b) Coordination environment of the aluminum atom. Selected atomic distances [pm]: Ge−C1 201.13(8), Ge−C4 200.87(7), Al−C1 215.11(7), Al−C2 230.90(9), Al−C3 230.40(9), Al−C4 214.56(10), Ge−Al 245.70(6), C1−C2 144.46(10), C2−C3 142.33(10), C3−C4 144.43(11), Al−Ct 190.14(6), Al−C(Cp*) 217–235, C(Cp*)−C(Cp*) 142–144.
Pure, solid germaaluminocenes 2 are thermally stable under inert conditions up to T=170 °C and do not react with common organic solvents, such as saturated and aromatic hydrocarbons, chlorinated aromatic hydrocarbons and ethers at room temperature. With methylene chloride, we observed slow decomposition to unidentified products. Also no reaction takes place with silanes (Et3SiH, PhSiH3) and dihydrogen.
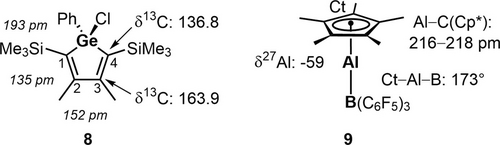
The reaction of germaaluminocenes 2 with tris(pentafluorophenyl)borane (BCF) as an example for a main group Lewis acid gave the expected Lewis acid base complexes 7 in acceptable to high yields (Scheme 4). The increased steric bulk of germaaluminocenes 2 b became noticeable by a significant longer reaction time t. The identity of the covalent germylborates 7 was established by NMR spectroscopy and was confirmed by a sc-XRD analysis of BCF complex 7 a. One set of signals in the 1H and 13C NMR spectra in the typical chemical shift range for the cyclopentadienyl groups suggest η5-coordination of the aluminum atom (δ1H=1.69 (7 a), 1.87 (7 b), δ13C=9.4 (7 a), 10.5 (7 b) (Cp*-CH3) and δ13C=117.2 (7 a), 114.7 (7 b) (Cp*-C), Table S13). The 13C NMR chemical shifts of the ring carbon atoms of the germole ring in the aluminocene complexes 7 (δ13C(1/4)=146.1 (7 a), 146.5 (7 b), δ13C(2/3)=163.1 (7 a), 162.7 (7 b), Table S13) are very close to related germole derivatives with tetracoordinated germanium atoms (e.g. chlorophenylgermole 8, see Scheme 5) and indicate a localized butadiene system.27, 28 The 11B NMR parameters for the BCF complex 7 a are characteristic for tetracoordinated boron centers (δ11B=−13.8, ω1/2=260 Hz). The tetrahedral coordination environment of the boron atoms in complexes 7 is also suggested by the small 19F NMR chemical shift difference detected for the fluorine atoms in the para- and meta- positions of the pentafluorophenyl substituent Δδ19Fm,p (Δδ19Fm,p=3.89 (7 a)).29 This value is very similar to the value found for the borate anion [B(C6F5)4]− with a tetracoordinated boron atom. For trigonal coordinated borane BCF a much larger Δδ19Fm,p is reported (Δδ19Fm,p=18.20 (BCF), vs. 3.80 [B(C6F5)4]−).
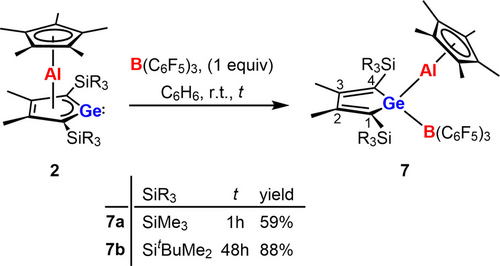
Reaction of germaaluminocenes 2 with tris(pentafluorophenyl)borane.
Compounds relevant for the discussion.
Colorless crystals of the BCF complex 7 a, suitable for sc-XRD analysis, were obtained directly from benzene solution. Its molecular structure in the crystal shows all general features derived from the NMR spectroscopic results. The addition of the tris(pentafluorophenyl)boranyl substituent leads to a tetracoordinated germanium atom, which is then linked to the η5-cyclopentadienylaluminyl group via a Ge−Al bond (Figure 3). The Ge−B bond (218.1 pm) is slightly longer than found for germyltriphenylborate anions (e.g. 213.8 pm for [Li(dme)2][Ph2MeGe−BPh3).30 The sum of the bond angles around the boron atom (Σ(C−B−C)=335.1°) indicates significant distortion from a trigonal planar coordination environment, which is larger than that reported for the Cp*Al donor acceptor complex 9 (Σ(C−B−C)=339.8°).31 The long Ge−C1/4 bonds and the significant short-long-short variation of the inner-cyclic C−C bonds indicate the localized structure of the germole ring (see Table S14). The Ge−Al bond (247.7 pm) is almost of the same length as in the aluminocenes 2 (248.8 pm (2 a),11 245.7 pm (2 b)) and shorter than reported for germylalanes and germylalanates (251–255 pm).32, 33 The Al−C(Cp*) distances fall in a very small range (216–219 pm) and indicate η5-coordination. The almost linear arrangement of the center of the Cp*-ring and the aluminum and germanium atoms is reminiscent of of the similar rearrangement of the Al and B atoms and the Cp*ring in the donor-acceptor complex 9.31
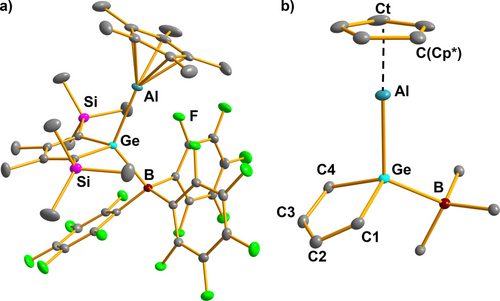
a) Molecular structure of the tris(pentafluorophenyl)borane complex 7 a in the crystal (Hydrogen atoms are omitted, thermal ellipsoids with 50 % probability). b) Coordination environments of the aluminum and germanium atoms. Selected atomic distances [pm] and angles [°]: Ge−C1 196.84(11), Ge−C4 197.37(12), Ge−Al 247.66(5), Ge−B 218.10(13), C1−C2 135.94(16), C2−C3 149.88(16), C3−C4 136.10(15), Al−Ct 180.06(4), Al−C(Cp*) 216–219, C(Cp*)−C(Cp*) 143–144; C1−Ge−C4 90.749(47), B−Ge−Al 120.509(35), Ge−Al−Ct 177.498(22).
The reactivity of germaaluminocenes 2 with nucleophiles is less clear. With Et3P in toluene no reaction occurred, even at 95–100 °C for 4 h. With Et3PO at room temperature immediate decomposition took place. In the reaction mixture only Cp*H was identified by 1H NMR spectroscopy. In contrast, a selective reaction takes place with one equivalent of tetramethylimidazolidine (Me4Im).34 Both germaaluminocenes 2 give the corresponding N-heterocyclic carbene (NHC) complexes 10 in high yields (Scheme 6). Addition of excess of Me4Im (3 equiv.) to germaaluminocene 2 a leads, however, to a complex product mixture. At room temperature, 1H and 13C NMR spectra of the isolated complexes 10 show only one set of signals for the Cp* substituent (see Table S13). The resonance frequencies of the carbenic carbon atoms were detected by 1H/13C HMBC experiments as cross signals with the N-methyl protons and are significantly shifted to lower frequencies compared to the free carbene (δ13C(NCN)=165.0 (10 a), 164.4 (10 b) vs. 213 (free Me4Im)).34 The carbon atoms of the germole ring of complexes 10 are almost isochrone (δ13C(1/4)=145.3 (10 b); δ13C(2/3)=145.1 (10 a), 146.9 (10 b)).35 This indicates a bonding situation, which is different from that of germaaluminocenes 2 and their complexes 7 with the Lewis acid BCF.

Reaction of germaaluminocenes 2 with a Lewis basic N-heterocyclic carbene.
Crystalline material, suitable for sc-XRD analysis, was obtained for both complexes 10 a and 10 b from saturated solutions in benzene at room temperature. Both structure solutions are almost identical and only the molecular structure of 10 a (Figure 4) will be discussed here (see Supporting Information material for structural details of complex 10 b). The molecular structure of 10 a differs significantly from those of germaaluminocenes 2 and from that of the BCF adduct 7 a. The carbene is bound to the aluminum center in an anti-orientation to the germanium atom. The carbene C−Al bond length is 204.8 pm, which is at the short end of a typical Al−C(carbene) distance of Al(III)-carbene complexes (205–216 pm, 212 pm in Me2iPr2Im−AlMe3).36, 37 The electron donation from the carbene to the aluminum center changes the coordination of the Cp*-ligand from η5 to η1 (Al−Cα=211 pm, see also Table S14). All Al−C contacts to the germole ring in the NHC complex 10 a (Al−C1/4=217.9, 219.4 and Al−C2/3=240.0, 241.8 pm) are significantly larger than in germaaluminocene 2 a, which shows shorter distances to the carbon atoms C1/4. The innercyclic C−C bonds fall all in a small range (139.4–146.5 pm) but show a marked trochaic (long-short-long) variation. Finally, also the Ge−Al distance (257.5 pm) is elongated by the coordination of the carbene, it is however only slightly longer than Ge−Al bonds of germylalanes and germylalanates (251–255 pm).32, 33
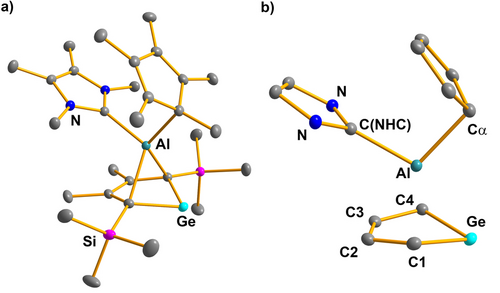
a) Molecular structure of the N-heterocyclic carbene complex 10 a in the crystal (Hydrogen atoms are omitted, thermal ellipsoids with 50 % probability). b) Coordination environment of the aluminum atom. Selected atomic distances [pm] and angles [°]: Ge−C1 199.10(11), Ge−C4 198.99(14), Al−C1 217.88(12), Al−C2 240.02(12), Al−C3 241.77(12), Al−C4 219.36(10), Ge−Al 257.50(5), C1−C2 146.16(19), C2−C3 139.39(15), C3−C4 146.46(16), Al−C(NHC) 204.80(13), Al−Cα 210.95(12), C1−Al−C4 74.144(43), C(NHC)−Al−Cα 102.695(47).
Next, we wanted to test the behavior of germaaluminocenes 2 versus subsequent addition of Lewis acids and bases. Therefore, we added one equivalent of Me4Im to the freshly synthesized BCF complex 7 a. This reaction was not selective and among several other products the germaaluminocene 2 a and unreacted complex 7 a were identified by NMR spectroscopy. We then applied electrophilic reagents E−X such as MeI, MeOTf and Me3SiOTf (OTf=triflate, OSO2CF3). After transferring the electrophile E+ to the germaaluminocene, these reagents provide an X− anion of only moderate nucleophilicity, which could add to the electrophilic center of the intermediates [11]+ or [12]+ (Scheme 7). Thus, in the reaction versus aluminocenes 2, reagents E−X would therefore mimic an amphiphile.
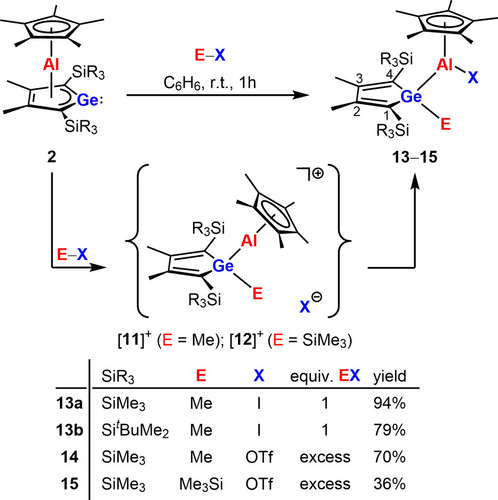
Reaction of germaaluminocenes 2 with amphiphiles MeI, MeOTf and Me3SiOTf (OTf=triflate, OSO2CF3).
Indeed, the reactions of both germaaluminocenes 2 a,b with MeI give the covalent aluminum iodides 13, and with MeOTf and Me3SiOTf 2 a gives the covalent aluminum esters 14 and 15 in high to sufficient isolated yields (Scheme 7). All compounds 13, 14, 15 were characterized by NMR spectroscopy, HRMS and in the case of 13 and 15 also by sc-XRD analysis. In the 1H NMR spectra of the germoles 13–15, the resonances of the methyl- (13, 14) or trimethylsilyl- (15) groups bound to the germanium atoms appear in the expected low frequency region (δ1H(Me)=0.59–0.71, δ1H(SiMe3)=0.28). 1H NMR and 13C NMR data of 13–15 are consistent with η5-coordination of the Cp* ring to the aluminum atom (δ1H(Cp*)=1.74–1.80; δ13C(Cp*)=116.3–117.4, 10.2–11.4). In addition, the 27Al NMR parameters (δ27Al=−31.8–−43.6, ω1/2=6–7 kHz) agree with this coordination mode.38 The deshielded 13C NMR resonances at δ13C=148.8–150.5 (C(1/4) and δ13C=160.4–160.9 (C2/3) are indicative for the localized butadiene systems in the germoles 13–15 and are similar to the data obtained for the BCF complexes 7 (see Table S13). The specific 13C NMR chemical shift of the CF3 group (δ13C(CF3)=119.7 (14) and 119.9 (15)) indicate that in solution both triflates, 14 and 15, are bound covalently to the aluminum atom. Ionic triflates are distinguished by a slightly but typically more deshielded 13C nucleus of the CF3 group (see Scheme 8 for comparison of triflates 16 and [17][OTf]).27
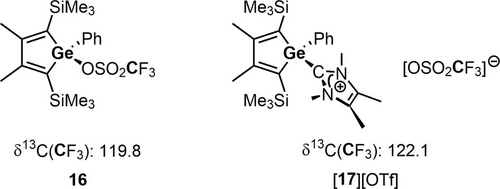
Comparison of 13C NMR chemical shifts of the CF3 group of the covalent germolyl triflate 16 and the ionic triflate [17][OTf].27
When we changed the applied silyl Lewis acid from silyl triflate to the triethylsilylbenzenium borate [Et3Si(C6H6)][B(C6F5)4], the reaction with the aluminocene 2 a stopped at the stage of the ionic intermediate (Scheme 7) and we identified the borate [18][B(C6F5)4] by NMR spectroscopy (Scheme 9). The 1H and 13C NMR parameters of cation [18]+ are close to that of the BCF complexes 7 (Scheme 4, Table S13). Unlike the BCF complexes 7, which do not show 27Al NMR signals, a broad 27Al resonance of cation [18]+ at a low frequency (δ27Al=−53.4, ω1/2=16 kHz) supports the η5-coordination of the aluminum atom (e.g. δ27Al=−59.4, ω1/2=1.5 kHz reported for BCF complex of Cp*Al(I), 9).31 The 29Si NMR chemical shift of the Et3Si substituent (δ29Si=14.4) in [18]+ is shifted to higher frequencies compared to silylated germoles (δ29Si=−7.6–−7.9).28 This chemical shift region is typical for silyl cations stabilized by main group donors, such as phosphanes.39 In addition, NMR chemical shift computations suggest for DFT-optimized structure of cation [18]+ a 27Al NMR chemical shift of δ27Al=−47 and a 29Si NMR chemical shift of δ29Si=0.0 (at M06-L/6-311G(2d,p)//M06-2X/6-311+G(d,p)).40

Reaction of germaaluminocene 2 a with [Et3Si(C6H6)][B(C6F5)4].
Crystals suitable for sc-XRD were obtained from saturated solutions of iodides 13 and ester 15 in nhexane at −20 °C. While the quality of the structure solution for 15 is not sufficient and confirms only the identity and the topology of the compound (see Supporting Information material, Figure S108), the structure solutions of iodides 13 allow a detailed analysis of their molecular structures. Both are very similar and the structure of the trimethylsilyl derivative 13 a is shown in Figure 5 and will be discussed in more detail. The data for 13 b is summarized in the Supporting Information material. The metrics of the germole ring in 13 a are very close to that of the BCF complex 7 a, i.e. the germanium atom is tetracoordinated and the germole ring shows the characteristic iambic (short-long-short) alternation of the C−C bonds of the localized diene system (see Table S14). The aluminum atom is connected to the germanium center via a single bond (Ge−Al=248.1 pm) and η5-coordinated by the Cp* substituent. The additional bond to the iodine atom leads to a bending of the Ge−Al−Ct(Cp*) angle compared to 7 a (Ge−Al−Ct(Cp*)=130.2° (13 a) vs. 177.5 (7 a)) and to a larger separation between the aluminum atom and the Cp* ring atoms (Al−C(Cp*)=217–232 pm). The Al−I bond (261.2 pm) is slightly longer than found for η1-Cp*AlI2OEt2 (255 pm)41 but close to that found in (η5-Cp*AlI)2 (264 pm).42 Overall the sc-XRD data agrees with the conclusion drawn from the NMR spectroscopic results.
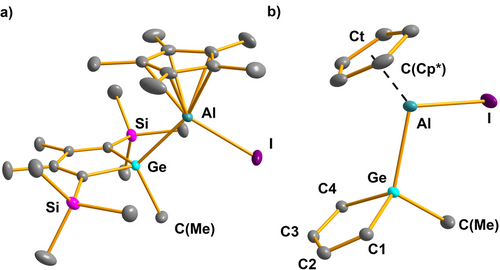
a) Molecular structure of the aluminum iodide 13 a in the crystal (Hydrogen atoms are omitted, thermal ellipsoids with 50 % probability). b) Coordination environment of the aluminum atom. Selected atomic distances [pm] and angles [°]: Ge−C1 195.82(12), Ge−C4 195.84(14), Ge−Al 248.08(5), Ge−C(Me) 196.68(15), C1−C2 135.76(20), C2−C3 150.09(17), C3−C4 135.72(19), Al−I 261.20(5), Al−Ct 188.10(4), Al−C(Cp*) 217–232, C(Cp*)−C(Cp*) 141–144; C1−Ge−C4 90.464(53), C(Me)−Ge−Al 110.070(46), Ge−Al−Ct 130.166(19).
Based on our results with alkyl iodides and triflates, we expected from the reaction of germaaluminocenes 2 with benzophenone the products of formal 1,2 additions, 2-alumina-1-germ-3-oxetanes. According to NMR spectroscopy, these reactions proceed quantitatively at room temperature. In each case we obtained only one single product in high isolated yields, which was identified as the expected germetane 19. Excess of ketone resulted in inseparable mixtures of unidentified products. The germetanes 19 were identified by NMR spectroscopy and sc-XRD analysis. In general, the NMR data is very close to that of the methyl iodide addition product 13 a (see Table S13). Characteristic for the germetane rings are the 13C NMR resonances at δ13C(OCPh2)=79.5 (19 a) and 77.0 (19 b). The 13C NMR chemical shifts of the germole carbon atoms are as expected for a localized butadiene system (δ13C=142.0 (19 a) and 142.9 (19 b) (C(1/4) and δ13C=160.1 (19 a) and 160.9 (19 b) (C2/3)). The η5-coordination of the aluminum atoms to the Cp* rings is indicated by two 13C NMR signals at δ13C(Cp*)=114.3 (19 a) and 114.7 (19 b) and δ13C(Cp*-CH3)=10.3 (19 a) and 10.6 (19 b) and a 27Al resonance at δ27Al=−25.3, ω1/2=8 kHz (19 a) and =−, ω1/2=9 kHz (19 b) (see Table S13).
Suitable crystals for sc-XRD of both germetanes 19 were obtained by recrystallization from nhexane. The structure of the trimethylsilyl derivative 19 a is shown in Figure 6 and will be discussed in more detail. The data for 19 b is summarized in the Supporting Information material. The central motif of the molecular structure of 19 a is the strained four-membered GeAlOC ring with a tetracoordinated germanium atom in a distorted tetrahedral coordination environment (see Figure 6). The aluminum atom is η5-coordinated by the Cp* substituent and connected via a regular Ge−Al single bond (244.1 pm vs. standard 247 pm) and a short Al−O bond (175.1 pm vs. standard 189 pm) to its neighbors. The innercyclic C−O bond (143.3 pm vs. standard 138 pm) and Ge−C bond (208.3 pm vs. standard 196 pm) are long to compensate for the ring strain of the four-membered cycle.26 The germole ring shows the expected localized butadiene system (see Figure 6 and Table S14).
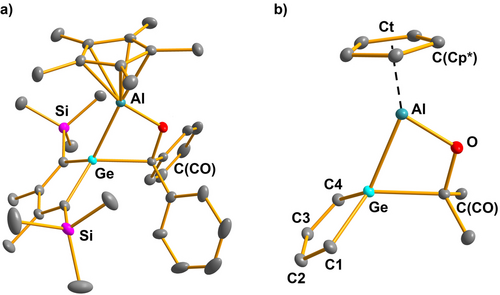
a) Molecular structure of the germetane 19 a in the crystal (Hydrogen atoms are omitted, thermal ellipsoids with 50 % probability). b) Coordination environment of the aluminum atom. Selected atomic distances [pm] and angles [°]: Ge−C1 195.61(7), Ge−C4 195.71(7), Ge−Al 244.14(4), Ge−C(CO) 208.33(7), C(CO)−O 143.33(7), C1−C2 136.04(9), C2−C3 150.48(10), C3−C4 136.06(9), Al−O 175.10(6), Al−Ct 183.62(3), Al−C(Cp*) 217–223, C(Cp*)−C(Cp*) 143–144; C1−Ge−C4 91.181(30), C(CO)−Ge−Al 70.051(21), Ge−Al−Ct 149.371(18).
Finally, the reactivity of germaaluminocenes 2 versus electron rich transition metal complexes was studied. We chose bis(cyclooctadiene) nickel(0) (Ni(COD)2) as reactant and we isolated orange COD nickel complexes 20 a,b using a 1 : 1 stoichiometry (Scheme 10). These compounds are stable in the solid state but decompose in benzene solution over time. In the case of 2 a a dark orange precipitate forms and XRD analysis of selected single crystals indicated decomposition of 20 a with formation of the dinuclear COD-bridged nickel(0) complex 20 c (see Supporting Information material for the results of the sc-XRD analysis). The NMR data of 20 a are consistent with the proposed composition as the 1H NMR spectra show the Cp* protons, protons of the substituents at the germole and those of the COD ligand in relative ratio of 1 : 1 : 1. The COD ligand gives rise to five multiplets in the 1H NMR spectrum (δ1H=5.73, 3.32, 2.38, 2.14, 1.80) and in the 1H/13C HMQC NMR spectrum to four correlated 13C NMR resonances (δ13C=130.6, 61.2, 33.8, 31.7). The η5-coordination of the Cp* ligand to the aluminum center in solution is indicated by only one signal for each magnetically different nucleus in the 1H and 13C NMR spectra (δ1H=1.90; δ13C=115.8, 10.4). The NMR signals of the carbon atoms of the germole ring are shifted to lower frequencies (δ13C=129.5 (C1/4), δ13C=139.7 (C2/3)) compared to other localized butadiene systems e.g. the methyl germoles 13 (Scheme 7).
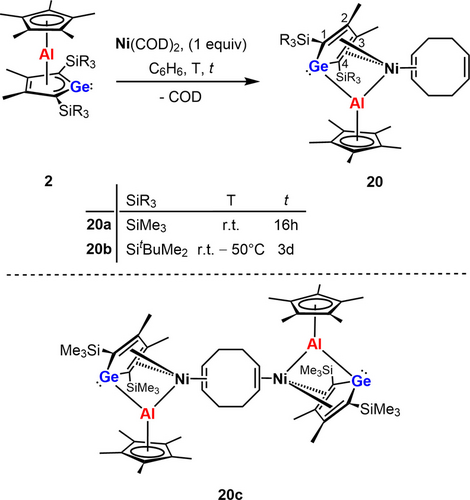
Reaction of germaaluminocenes 2 with Ni(COD)2.
Crystals of the nickel complexes 20 suitable for sc-XRD were obtained from benzene (20 a) or nhexane (20 b). The structure of the trimethylsilyl derivative 20 a is shown in Figure 7 and will be discussed in more detail. The data for 20 b is summarized in the Supporting Information material. The germaaluminocene molecule operates in the mononuclear Ni(0) complex 20 a as tridentate ligand. The coordination environment of the nickel atom is similar to that in related η4-heterole complexes such as 21 and 22 and typical for butadiene complexes of late transition metals (Scheme 11). The dicoordinated germanium atom is bent away from the nickel center, the folding angle α as measured by the dihedral angle between the plane of the butadiene part and the plane spanned by the atoms GeC1 C4, is 10.0°. This folding is less developed than in the η4-complexes 21 and 22 complexes with tetracoordinated tetrel atoms.44, 45 Nevertheless, the Ni/Ge distance (248.6 pm) in 20 a is larger than found in germylene- 23 and germylnickel complexes 24 and suggests no direct bonding interaction between these two centers.46 The Ge−Al bond of the germaaluminocene part in 20 a is elongated to 262.5 pm, and also the Cp*Al−Ni separation (231.4 pm) is larger than found in the homoleptic nickel aluminum complex 25 (Scheme 11).47, 48 These metrics around the GeAlNi core of complex 20 a suggest an electron-deficient multicenter bonding between these three atoms.
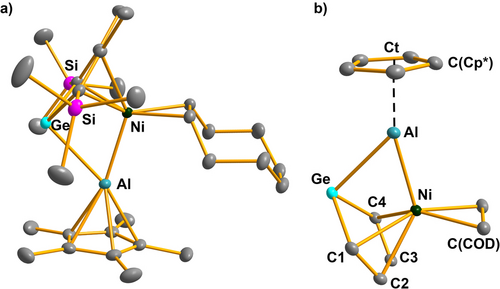
a) Molecular structure of the nickel complex 20 a in the crystal. (Hydrogen atoms are omitted, thermal ellipsoids with 50 % probability). b) Coordination environment of the aluminum atom. Selected atomic distances [pm] and angles [°]: Ge−C1 199.54(6), Ge−C4 199.92(8), Ge−Al 262.47(4), Ge−Ni 248.62(4), Al−Ni 231.42(4), Ni−C(germole) 216–218, C1−C2 140.18(10), C2−C3 145.73(9), C3−C4 140.30(8), Al−Ct 188.93(3), Al−C(Cp*) 224–225, C(Cp*)−C(Cp*) 142–143; C1−Ge−C4 85.208(26), Ni−Ge−Al 53.763(10), Ge−Al−Ct 133.095(13), Ni−Al−Ct 166.814(16), folding angle of the germole ring α=10.0°.
The distinct reactivity of germaaluminocenes 2 calls for an analysis of their electronic situation. For this purpose, we used density functional theory (DFT) calculations based on optimized molecular structures at the M06-2X/6-311+G(d,p) level.40 This combination of basis set and DFT method provides molecular structures whose structural parameters are close to the experimental data (main deviation in bond lengths 1.5 %, see Table S15) The frontier molecular orbitals (FMOs) of germaaluminocene 2 a are dominated by contributions from orbitals of the aluminum atom and the germole ring (Figure 8). The HOMO is a π-type orbital of the germole ring with significant additions of atomic orbitals of the aluminum center. The HOMO-1 and HOMO-2 are almost degenerate in energy and represent linear combinations of π-type orbitals of the butadiene part of the germole ring, of π-type orbitals of the Cp* substituent and of the in-plane lone pair at the germanium atom. Also, the LUMO is a π-orbital of the germole ring, which is strongly polarized towards the germanium atom and the LUMO+1 is mainly located at the aluminum center. The chemical bonding in germaaluminocenes 2 is characterized by strong delocalization of electrons between the aluminum atom and the germole ring. This is further substantiated by the results of the natural bond orbital (NBO) analysis,43 which suggest a bicyclic structure 2 a(A) with significant delocalization of the 6 electrons of the two Al−C1/C4 bonds and the C2=C3 π-bond across the 6 atoms of the C4GeAl pentagonal pyramid (see Figure 9 and Figure S119). This results in considerable covalent bonding between the aluminum center and all 5 atoms of the germole ring as indicated by the computed Wiberg bond indices (WBI) (Figure 9). Based on this analysis a representation of germaaluminocene 2 a as Cp*-substituted nido-cluster 2 a(B) is justified, in agreement with the skeletal electron count (16 electrons for a nido-structure built from 6 cluster atoms, Supporting Information material, page S94). Consequently, the separation of germaaluminocene 2 a into the two neutral units, Cp*Al(I) and the cyclic germol-1-ylene is strongly endothermic by 200 kJ mol−1.
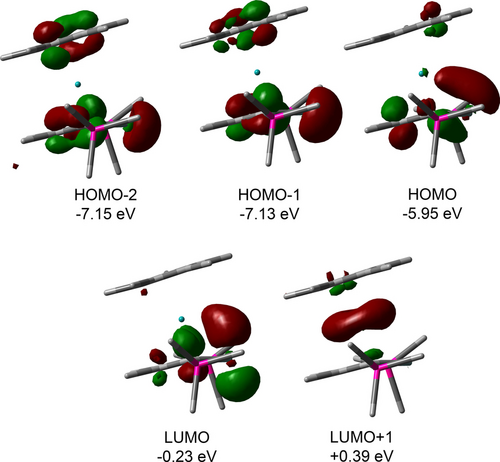
Calculated surface diagrams of the frontier molecular orbitals (FMOs) of germaaluminocene 2 a (M06-2X/def2-tzvp//M06-2X/6-311+G(d,p), isodensity value 0.055, hydrogen atoms are omitted).

Preferred Lewis structure 2 a (A) and its representation as nido-cluster 2 a (B) based on natural bond orbital (NBO) analysis of germaaluminocene 2 a. Calculated Wiberg bond indices (WBIs) at M06-2X/def2tzvp//M06-2X/6-311+G(d,p), data for Me2AlGeMe3 and Me3Al are given on the right side.
Based on these frontier orbitals, the reactivity of germaaluminocenes 2 can be rationalized. Nucleophiles attack the aluminum center, while hard electrophiles add to germanium atom. The π-system of the germole ring offers an alternative reaction side for soft Lewis acids, such as electron rich metal centers. Reactants with polar bonds and polar multiple bonds behave accordingly and undergo a formal addition across the Ge−Al bond. Common to these reactions, but rather unexpected, is the conservation of the Ge−Al bond in all products, although it is elongated in the NHC complexes 10 (256, 258 pm vs. Ge−Al (mean)=252 pm).46 In the case of the Ni(0) complexes 20, it is part of a multicenter bond, which again leads to a long Ge−Al linkage (Ge−Al: 262–267 pm). The cyclopentadienyl substituent at the aluminum atom is beneficial as it helps to accommodate very diverse electronic situations at the aluminum center by changing its hapticity.
Conclusion
Germaaluminocenes 2 are amphiphiles with an electrophilic aluminum center and a dicoordinated nucleophilic germanium center. Reactions of the sandwich compounds 2 with a Lewis acid give germoles with a tetracoordinated germanium atom and a η5-Cp*Al substituent, e.g. the BCF complexes 7 and the silylated cation [18]+. Structurally, those compounds show a close resemblance to the donor-acceptor stabilized germylenes popularized by the Rivard group.49, 50 Nucleophiles add to the aluminum atom and the hapticity of the Cp*-substituent changes from η5 to η1. With ketones, germaaluminocenes 2 give four-membered Ge−Al−O−C heterocycles. The comparison of the reactivity pattern of germaaluminocenes 2 described here with the expected pattern of hypothetical aluminagermapentafulvenes, compounds that are not even minima on the potential energy surface,11 reveals a striking similarity (Figure 10). Although quite different in the molecular structures, the reactive sides of both compounds behave similar and the structure of the products such as adducts of Lewis acids 7, and [18]+, the NHC-complexes 10, the addition products of amphiphiles 13–15, the germetanes 19 and the nickel(0) complexes 20, each composed of a substituted germole ring and an aluminocene part with different hapticity, are clearly derived from the pentafulvene. In this respect, germaaluminocenes 2 are synthetic equivalents for the non-existing pentafulvenes.
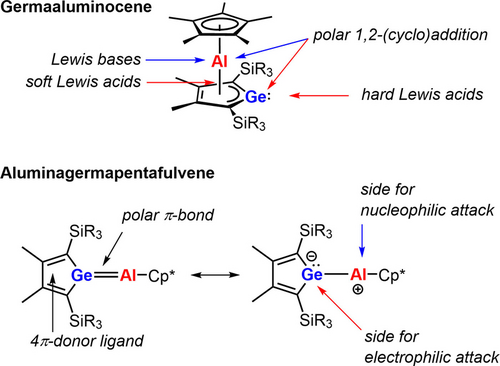
Comparison of the reactivity pattern of germaaluminocenes 2 and the expected reactive sides of the non-existent aluminagermapentafulvene.
Supporting Information
The authors have cited additional references within the Supporting Information.51-58
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) (MU-1440/13-1 and INST 184/227-1). Computations were done at the HPC Cluster CARL, University of Oldenburg, funded by the DFG (INST 184/108-1 FUGG) and the Ministry of Science and Culture (MWK) of the Lower Saxony State. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.