Overcoming Electrostatic Interaction via Pulsed Electroreduction for Boosting the Electrocatalytic Urea Synthesis
Weibin Qiu
College of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou, 341000 PR China
These authors contributed equally to this work.
Search for more papers by this authorShimei Qin
College of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou, 341000 PR China
These authors contributed equally to this work.
Search for more papers by this authorYibao Li
College of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou, 341000 PR China
Search for more papers by this authorNing Cao
College of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou, 341000 PR China
Search for more papers by this authorWeirong Cui
College of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou, 341000 PR China
Search for more papers by this authorZedong Zhang
Department of Chemistry, Tsinghua University, Beijing, 100084 P. R. China
Search for more papers by this authorZechao Zhuang
Department of Chemistry, Tsinghua University, Beijing, 100084 P. R. China
Search for more papers by this authorCorresponding Author
Dingsheng Wang
Department of Chemistry, Tsinghua University, Beijing, 100084 P. R. China
Search for more papers by this authorCorresponding Author
Yong Zhang
College of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou, 341000 PR China
Search for more papers by this authorWeibin Qiu
College of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou, 341000 PR China
These authors contributed equally to this work.
Search for more papers by this authorShimei Qin
College of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou, 341000 PR China
These authors contributed equally to this work.
Search for more papers by this authorYibao Li
College of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou, 341000 PR China
Search for more papers by this authorNing Cao
College of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou, 341000 PR China
Search for more papers by this authorWeirong Cui
College of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou, 341000 PR China
Search for more papers by this authorZedong Zhang
Department of Chemistry, Tsinghua University, Beijing, 100084 P. R. China
Search for more papers by this authorZechao Zhuang
Department of Chemistry, Tsinghua University, Beijing, 100084 P. R. China
Search for more papers by this authorCorresponding Author
Dingsheng Wang
Department of Chemistry, Tsinghua University, Beijing, 100084 P. R. China
Search for more papers by this authorCorresponding Author
Yong Zhang
College of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou, 341000 PR China
Search for more papers by this authorGraphical Abstract
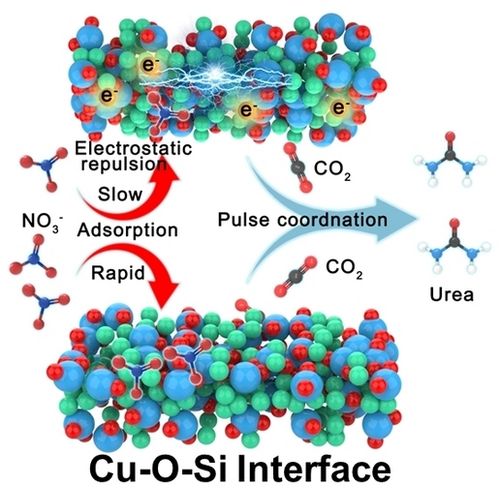
The electrocatalytic C−N coupling from carbon dioxide and nitrate is a sustainable and promising alternative for urea synthesis. We developed a stable CuSiOx catalyst with abundant atomic Cu−O−Si interfacial sites toward urea electrosynthesis, in which Cu species are uniformly dispersed in the silica matrix. Besides, we adopt pulsed electroreduction to overcome electrostatic interaction, promoting nitrate adsorption and reduction to urea.
Abstract
Electrocatalytic urea synthesis under ambient conditions offers a promising alternative strategy to the traditional energy-intensive urea industry protocol. Limited by the electrostatic interaction, the reduction reaction of anions at the cathode in the electrocatalytic system is not easily achievable. Here, we propose a novel strategy to overcome electrostatic interaction via pulsed electroreduction. We found that the reconstruction-resistant CuSiOx nanotube, with abundant atomic Cu−O−Si interfacial sites, exhibits ultrastability in the electrosynthesis of urea from nitrate and CO2. Under a pulsed potential approach with optimal operating conditions, the Cu−O−Si interfaces achieve a superior urea production rate (1606.1 μg h−1 mgcat.−1) with high selectivity (79.01 %) and stability (the Faradaic efficiency is retained at 80 % even after 80 h of testing), outperforming most reported electrocatalytic synthesis urea catalysts. We believe our strategy will incite further investigation into pulsed electroreduction increasing substrate transport, which may guide the design of ambient urea electrosynthesis and other energy conversion systems.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202402684-sup-0001-misc_information.pdf5.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aY. Huang, R. Yang, C. Wang, N. Meng, Y. Shi, Y. Yu, B. Zhang, ACS Energy Lett. 2021, 7, 284–291;
- 1bJ. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont, W. Winiwarter, Nat. Geosci. 2008, 1, 636–639.
- 2M. Xia, C. Mao, A. Gu, A. A. Tountas, C. Qiu, T. E. Wood, Y. F. Li, U. Ulmer, Y. Xu, C. J. Viasus, J. Ye, C. Qian, G. Ozin, Angew. Chem. Int. Ed. 2021, 61, e202110158.
- 3
- 3aV. Kyriakou, I. Garagounis, A. Vourros, E. Vasileiou, M. Stoukides, Joule 2020, 4, 142–158;
- 3bC. Chen, X. Zhu, X. Wen, Y. Zhou, L. Zhou, H. Li, L. Tao, Q. Li, S. Du, T. Liu, D. Yan, C. Xie, Y. Zou, Y. Wang, R. Chen, J. Huo, Y. Li, J. Cheng, H. Su, X. Zhao, W. Cheng, Q. Liu, H. Lin, J. Luo, J. Chen, M. Dong, K. Cheng, C. Li, S. Wang, Nat. Chem. 2020, 12, 717–724.
- 4H. Song, D. A. Chipoco Haro, P.-W. Huang, L. Barrera, M. C. Hatzell, Acc. Chem. Res. 2023, 56, 2944–2953.
- 5C. Lv, L. Zhong, H. Liu, Z. Fang, C. Yan, M. Chen, Y. Kong, C. Lee, D. Liu, S. Li, J. Liu, L. Song, G. Chen, Q. Yan, G. Yu, Nat. Sustainability 2021, 4, 868–876.
- 6
- 6aD. Zhang, Y. Xue, X. Zheng, C. Zhang, Y. Li, Natl. Sci. Rev. 2023, 10, nwac209;
- 6bX. Liu, Y. Jiao, Y. Zheng, M. Jaroniec, S. Z. Qiao, Nat. Commun. 2022, 13, 5471;
- 6cX. Zhang, X. Zhu, S. Bo, C. Chen, M. Qiu, X. Wei, N. He, C. Xie, W. Chen, J. Zheng, P. Chen, S. P. Jiang, Y. Li, Q. Liu, S. Wang, Nat. Commun. 2022, 13, 5337;
- 6dD. Li, N. Xu, Y. Zhao, C. Zhou, L. P. Zhang, L. Z. Wu, T. Zhang, Small Methods 2022, 6, e2200561;
- 6eJ. Geng, S. Ji, M. Jin, C. Zhang, M. Xu, G. Wang, C. Liang, H. Zhang, Angew. Chem. Int. Ed. 2023, 62, e202210958;
- 6fC. S. Gerke, M. Klenk, P. Zapol, V. S. Thoi, ACS Catal. 2023, 13, 14540–14547;
- 6gC. S. Gerke, Y. Xu, Y. Yang, G. D. Foley, B. Zhang, E. Shi, N. M. Bedford, F. Che, V. S. Thoi, J. Am. Chem. Soc. 2023, 145, 26144–26151;
- 6hX. Tu, X. Zhu, S. Bo, X. Zhang, R. Miao, G. Wen, C. Chen, J. Li, Y. Zhou, Q. Liu, D. Chen, H. Shao, D. Yan, Y. Li, J. Jia, S. Wang, Angew. Chem. Int. Ed. 2023, 62, e202317087.
- 7
- 7aN. Meng, X. Ma, C. Wang, Y. Wang, R. Yang, J. Shao, Y. Huang, Y. Xu, B. Zhang, Y. Yu, ACS Nano 2022, 16, 9095–9104;
- 7bY. Zhao, Y. Ding, W. Li, C. Liu, Y. Li, Z. Zhao, Y. Shan, F. Li, L. Sun, F. Li, Nat. Commun. 2023, 14, 4491;
- 7cY. Luo, K. Xie, P. Ou, C. Lavallais, T. Peng, Z. Chen, Z. Zhang, N. Wang, X.-Y. Li, I. Grigioni, B. Liu, D. Sinton, J. B. Dunn, E. H. Sargent, Nat. Catal. 2023, 6, 939–948;
- 7dJ. Leverett, T. Tran-Phu, J. A. Yuwono, P. Kumar, C. Kim, Q. Zhai, C. Han, J. Qu, J. Cairney, A. N. Simonov, R. K. Hocking, L. Dai, R. Daiyan, R. Amal, Adv. Energy Mater. 2022, 12, 2201500;
- 7eS. Liu, S. Yin, Z. Wang, Y. Xu, X. Li, L. Wang, H. Wang, Cell Rep. Phys. Sci. 2022, 3, 100869;
- 7fX. Wei, Y. Liu, X. Zhu, S. Bo, L. Xiao, C. Chen, T. T. T. Nga, Y. He, M. Qiu, C. Xie, D. Wang, Q. Liu, F. Dong, C.-L. Dong, X.-Z. Fu, S. Wang, Adv. Mater. 2023, 35, 2300020.
- 8
- 8aS. Popović, M. Smiljanić, P. Jovanovič, J. Vavra, R. Buonsanti, N. Hodnik, Angew. Chem. Int. Ed. 2020, 59, 14736–14746;
- 8bX. Tan, K. Sun, Z. Zhuang, B. Hu, Y. Zhang, Q. Liu, C. He, Z. Xu, C. Chen, H. Xiao, C. Chen, J. Am. Chem. Soc. 2023, 145, 8656–8664.
- 9
- 9aY. Wang, H. Li, W. Zhou, X. Zhang, B. Zhang, Y. Yu, Angew. Chem. Int. Ed. 2022, 61, e202202604;
- 9bY. Huang, C. He, C. Cheng, S. Han, M. He, Y. Wang, N. Meng, B. Zhang, Q. Lu, Y. Yu, Nat. Commun. 2023, 14, 7368.
- 10F. Yang, X. Mao, M. Ma, C. Jiang, P. Zhang, J. Wang, Q. Deng, Z. Zeng, S. Deng, Carbon 2020, 168, 528–535.
- 11
- 11aJ. Sun, J. Yu, Q. Ma, F. Meng, X. Wei, Y. Sun, N. Tsubaki, Sci. Adv. 2018, 4, eaau3275;
- 11bC. Xu, G. Chen, Y. Zhao, P. Liu, X. Duan, L. Gu, G. Fu, Y. Yuan, N. Zheng, Nat. Commun. 2018, 9, 3367;
- 11cT. W. van Deelen, C. Hernández Mejía, K. P. de Jong, Nat. Catal. 2019, 2, 955–970.
- 12L. He, Q. Wang, J. Feng, Z. Xie, J. Zhou, F. Fei, ChemElectroChem 2022, 9, e202200054.
- 13
- 13aR. Li, D. Wang, Nano Res. 2022, 15, 6888–6923;
- 13bL. Wang, J. Wu, S. Wang, H. Liu, Y. Wang, D. Wang, Nano Res. 2023;
- 13cT. Gan, D. Wang, Nano Res. 2024, 17, 18–38;
- 13dX. Zheng, J. Yang, P. Li, Z. Jiang, P. Zhu, Q. Wang, J. Wu, E. Zhang, W. Sun, S. Dou, D. Wang, Y. Li, Angew. Chem. Int. Ed. 2023, 62, e202217449.
- 14J. Jiao, R. Lin, S. Liu, W.-C. Cheong, C. Zhang, Z. Chen, Y. Pan, J. Tang, K. Wu, S.-F. Hung, H. M. Chen, L. Zheng, Q. Lu, X. Yang, B. Xu, H. Xiao, J. Li, D. Wang, Q. Peng, C. Chen, Y. Li, Nat. Chem. 2019, 11, 222–228.
- 15W.-H. Li, J. Yang, D. Wang, Angew. Chem. Int. Ed. 2022, 61, e202213318.
- 16Y. Yu, R. Jin, J. Easa, W. Lu, M. Yang, X. Liu, Y. Xing, Z. Shi, Chem. Commun. 2019, 55, 4178–4181.
- 17P. Dubot, D. Jousset, V. Pinet, F. Pellerin, J. P. Langeron, Surf. Interface Anal. 1988, 12, 99–104.
- 18N. Benito, M. Flores, J. Phys. Chem. C 2017, 121, 18771–18778.
- 19Y. Yu, Y. He, P. Yan, S. Wang, F. Dong, Proc. Nat. Acad. Sci. 2023, 120, e2307320120.
- 20H. Yang, L. Shang, Q. Zhang, R. Shi, G. I. N. Waterhouse, L. Gu, T. Zhang, Nat. Commun. 2019, 10, 4585.
- 21W. He, J. Zhang, S. Dieckhofer, S. Varhade, A. C. Brix, A. Lielpetere, S. Seisel, J. R. C. Junqueira, W. Schuhmann, Nat. Commun. 2022, 13, 1129.
- 22Y. Li, J. Ma, T. D. Waite, M. R. Hoffmann, Z. Wang, Environ. Sci. Technol. 2021, 55, 10695–10703.
- 23J. Shao, H. Jing, P. Wei, X. Fu, L. Pang, Y. Song, K. Ye, M. Li, L. Jiang, J. Ma, R. Li, R. Si, Z. Peng, G. Wang, J. Xiao, Nat. Energy 2023, 8, 1273–1283.
- 24
- 24aA. Bhardwaj, A. Kumar, H. Bae, C. J. Park, S. J. Song, J. Hazard. Mater. 2020, 396, 122601;
- 24bH. Du, H. Guo, K. Wang, X. Du, B. A. Beshiwork, S. Sun, Y. Luo, Q. Liu, T. Li, X. Sun, Angew. Chem. Int. Ed. 2023, 62, e202215782.
- 25W. Qiu, N. Yang, D. Luo, J. Wang, L. Zheng, Y. Zhu, E. M. Akinoglu, Q. Huang, L. Shui, R. Wang, G. Zhou, X. Wang, Z. Chen, Appl. Catal. B 2021, 293, 120216.
- 26J. Li, A. Ozden, M. Wan, Y. Hu, F. Li, Y. Wang, R. R. Zamani, D. Ren, Z. Wang, Y. Xu, D. H. Nam, J. Wicks, B. Chen, X. Wang, M. Luo, M. Graetzel, F. Che, E. H. Sargent, D. Sinton, Nat. Commun. 2021, 12, 2808.
- 27D. Zhu, L. Zhang, R. E. Ruther, R. J. Hamers, Nat. Mater. 2013, 12, 836–841.
- 28N. Salmon, R. Bañares-Alcántara, Nat. Synth. 2023, 2, 604–611.
- 29Y. Yang, L. Zhang, Z. Hu, Y. Zheng, C. Tang, P. Chen, R. Wang, K. Qiu, J. Mao, T. Ling, S.-Z. Qiao, Angew. Chem. Int. Ed. 2020, 59, 4525–4531.
- 30M. Xu, F. Wu, Y. Zhang, Y. Yao, G. Zhu, X. Li, L. Chen, G. Jia, X. Wu, Y. Huang, P. Gao, W. Ye, Nat. Commun. 2023, 14, 6994.
- 31Y. Zhao, F. Li, W. Li, Y. Li, C. Liu, Z. Zhao, Y. Shan, Y. Ji, L. Sun, Angew. Chem. Int. Ed. 2021, 60, 20331–20341.