Torsional Strain Enabled Ring-Opening Polymerization towards Axially Chiral Semiaromatic Polyesters with Chemical Recyclability
Qing Cao
National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), College of Chemistry, Sichuan University, 29 Wangjiang Rd, Chengdu, 610064 P. R. China
Search for more papers by this authorYi-Min Tu
National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), College of Chemistry, Sichuan University, 29 Wangjiang Rd, Chengdu, 610064 P. R. China
Search for more papers by this authorHua-Zhong Fan
National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), College of Chemistry, Sichuan University, 29 Wangjiang Rd, Chengdu, 610064 P. R. China
Search for more papers by this authorSi-Yi Shan
National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), College of Chemistry, Sichuan University, 29 Wangjiang Rd, Chengdu, 610064 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Zhongzheng Cai
National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), College of Chemistry, Sichuan University, 29 Wangjiang Rd, Chengdu, 610064 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Jian-Bo Zhu
National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), College of Chemistry, Sichuan University, 29 Wangjiang Rd, Chengdu, 610064 P. R. China
Search for more papers by this authorQing Cao
National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), College of Chemistry, Sichuan University, 29 Wangjiang Rd, Chengdu, 610064 P. R. China
Search for more papers by this authorYi-Min Tu
National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), College of Chemistry, Sichuan University, 29 Wangjiang Rd, Chengdu, 610064 P. R. China
Search for more papers by this authorHua-Zhong Fan
National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), College of Chemistry, Sichuan University, 29 Wangjiang Rd, Chengdu, 610064 P. R. China
Search for more papers by this authorSi-Yi Shan
National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), College of Chemistry, Sichuan University, 29 Wangjiang Rd, Chengdu, 610064 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Zhongzheng Cai
National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), College of Chemistry, Sichuan University, 29 Wangjiang Rd, Chengdu, 610064 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Jian-Bo Zhu
National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), College of Chemistry, Sichuan University, 29 Wangjiang Rd, Chengdu, 610064 P. R. China
Search for more papers by this authorGraphical Abstract
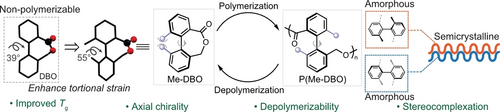
To overcome the non-polymerizability of the biaryl-fused monomer DBO, a cyclic ester Me-DBO installed with dimethyl substitution was prepared to enable its polymerizability via enhancing torsional strain while preserving an excellent recyclability. Remarkably, mixing these complementary enantiopure polymers containing axial chirality promoted a transformation from amorphous to crystalline material.
Abstract
The development of new chemically recyclable polymers via monomer design would provide a transformative strategy to address the energy crisis and plastic pollution problem. Biaryl-fused cyclic esters were targeted to generate axially chiral polymers, which would impart new material performance. To overcome the non-polymerizability of the biaryl-fused monomer DBO, a cyclic ester Me-DBO installed with dimethyl substitution was prepared to enable its polymerizability via enhancing torsional strain. Impressively, Me-DBO readily went through well-controlled ring-opening polymerization, producing polymer P(Me-DBO) with high glass transition temperature (Tg >100 °C). Intriguingly, mixing these complementary enantiopure polymers containing axial chirality promoted a transformation from amorphous to crystalline material, affording a semicrystalline stereocomplex with a melting transition temperature more than 300 °C. P(Me-DBO) were capable of depolymerizing back to Me-DBO in high efficiency, highlighting an excellent recyclability.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202400196-sup-0001-misc_information.pdf5.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1F. M. Haque, J. S. A. Ishibashi, C. A. L. Lidston, H. Shao, F. S. Bates, A. B. Chang, G. W. Coates, C. J. Cramer, P. J. Dauenhauer, W. R. Dichtel, C. J. Ellison, E. A. Gormong, L. S. Hamachi, T. R. Hoye, M. Jin, J. A. Kalow, H. J. Kim, G. Kumar, C. J. LaSalle, S. Liffland, B. M. Lipinski, Y. Pang, R. Parveen, X. Peng, Y. Popowski, E. A. Prebihalo, Y. Reddi, T. M. Reineke, D. T. Sheppard, J. L. Swartz, W. B. Tolman, B. Vlaisavljevich, J. Wissinger, S. Xu, M. A. Hillmyer, Chem. Rev. 2022, 122, 6322–6373.
- 2J.-G. Rosenboom, R. Langer, G. Traverso, Nat. Rev. Mater. 2022, 7, 117–137.
- 3H. S. Wang, N. P. Truong, Z. Pei, M. L. Coote, A. Anastasaki, J. Am. Chem. Soc. 2022, 144, 4678–4684.
- 4Q. Zhang, Y. Deng, C.-Y. Shi, B. L. Feringa, H. Tian, D.-H. Qu, Matter 2021, 4, 1352–1364.
- 5T. M. McGuire, A. Buchard, C. Williams, J. Am. Chem. Soc. 2023, 145, 19840–19848.
- 6X. Zhang, W. Guo, C. Zhang, X. Zhang, Nat. Commun. 2023, 14, 5423.
- 7H. G. Hester, B. A. Abel, G. W. Coates, J. Am. Chem. Soc. 2023, 145, 8800–8804.
- 8L. Zhou, Z. Zhang, C. Shi, M. Scoti, D. K. Barange, R. R. Gowda, E. Y.-X. Chen, Science 2023, 380, 64–69.
- 9Z. Li, D. Zhao, Y. Shen, Z. Li, Angew. Chem. Int. Ed. 2023, 62, e202302101.
- 10Y. Wang, Y. Zhu, W. Lv, X. Wang, Y. Tao, J. Am. Chem. Soc. 2023, 145, 1877–1885.
- 11J.-Z. Zhao, T.-J. Yue, B.-H. Ren, Y. Liu, W.-M. Ren, X.-B. Lu, Macromolecules 2022, 55, 8651–8658.
- 12R. M. Rapagnani, R. J. Dunscomb, A. A. Fresh, I. A. Tonks, Nat. Chem. 2022, 14, 877–883.
- 13C. Jehanno, J. W. Alty, M. Roosen, S. De Meester, A. P. Dove, E. Y.-X. Chen, F. A. Leibfarth, H. Sardon, Nature 2022, 603, 803–814.
- 14X.-L. Li, R. W. Clarke, J.-Y. Jiang, T.-Q. Xu, E. Y.-X. Chen, Nat. Chem. 2023, 15, 278–285.
- 15Y.-L. Su, L. Yue, H. Tran, M. Xu, A. Engler, R. Ramprasad, H. J. Qi, W. R. Gutekunst, J. Am. Chem. Soc. 2023, 145, 13950–13956.
- 16D. E. Fagnani, J. L. Tami, G. Copley, M. N. Clemons, Y. D. Y. L. Getzler, A. J. McNeil, ACS Macro Lett. 2021, 10, 41–53.
- 17X.-L. Li, K. Ma, F. Xu, T.-Q. Xu, Chem. – An Asian J. 2023, 18, e202201167.
- 18G. W. Coates, Y. D. Y. L. Getzler, Nat. Rev. Mater. 2020, 5, 501–516.
- 19M. Hong, E. Y.-X. Chen, Trends Chem. 2019, 1, 148–151.
- 20G. Xu, Q. Wang, Green Chem. 2022, 24, 2321–2346.
- 21J. Zhou, D. Sathe, J. Wang, J. Am. Chem. Soc. 2022, 144, 928–934.
- 22J. D. Feist, Y. Xia, J. Am. Chem. Soc. 2020, 142, 1186–1189.
- 23H.-Z. Fan, X. Yang, J.-H. Chen, Y.-M. Tu, Z. Cai, J.-B. Zhu, Angew. Chem. Int. Ed. 2022, 61, e202117639.
- 24G. X. De Hoe, T. Şucu, M. P. Shaver, Acc. Chem. Res. 2022, 55, 1514–1523.
- 25J. P. MacDonald, M. P. Shaver, Polym. Chem. 2016, 7, 553–559.
- 26H.-Z. Fan, X. Yang, Y.-C. Wu, Q. Cao, Z. Cai, J.-B. Zhu, Polym. Chem. 2023, 14, 747–753.
- 27E. Lizundia, V. A. Makwana, A. Larrañaga, J. L. Vilas, M. P. Shaver, Polym. Chem. 2017, 8, 3530–3538.
- 28L.-G. Li, Q.-Y. Wang, Q.-Y. Zheng, F.-S. Du, Z.-C. Li, Macromolecules 2021, 54, 6745–6752.
- 29M.-Q. Li, Z.-X. Luo, X.-Y. Yu, G.-Q. Tian, G. Wu, S.-C. Chen, Y.-Z. Wang, Macromolecules 2023, 56, 2465–2475.
- 30Y.-M. Tu, X.-M. Wang, X. Yang, H.-Z. Fan, F.-L. Gong, Z. Cai, J.-B. Zhu, J. Am. Chem. Soc. 2021, 143, 20591–20597.
- 31X.-M. Wang, H.-Y. Huang, Y.-M. Tu, Z. Cai, J.-B. Zhu, Polym. Chem. 2023, 14, 2027–2033.
- 32F. Ren, J. Xian, Z. Jia, Z. Chen, H. Fu, R. Wang, W.-D. Chu, X. Pan, J. Wu, Angew. Chem. Int. Ed. 2023, 62, e202306759.
- 33H. J. Kim, Y. Reddi, C. J. Cramer, M. A. Hillmyer, C. J. Ellison, ACS Macro Lett. 2020, 9, 96–102.
- 34X. Zhang, K. Zhao, Z. Gu, Acc. Chem. Res. 2022, 55, 1620–1633.
- 35G. Bringmann, A. J. Price Mortimer, P. A. Keller, M. J. Gresser, J. Garner, M. Breuning, Angew. Chem. Int. Ed. 2005, 44, 5384–5427.
- 36J. K. Cheng, S.-H. Xiang, S. Li, L. Ye, B. Tan, Chem. Rev. 2021, 121, 4805–4902.
- 37Y. Okamoto, Prog. Polym. Sci. 2000, 25, 159–162.
- 38T. Miao, X. Cheng, Y. Guo, G. Zhang, W. Zhang, Giant 2023, 14, 100161.
- 39H.-Y. Huang, W. Xiong, Y.-T. Huang, K. Li, Z. Cai, J.-B. Zhu, Nat. Catal. 2023, 6, 720–728.
- 40R. Röhrkasten, R. P. Kreher, Chem. Ber. 1991, 124, 2085–2090.
- 41B. M. Chamberlain, M. Cheng, D. R. Moore, T. M. Ovitt, E. B. Lobkovsky, G. W. Coates, J. Am. Chem. Soc. 2001, 123, 3229–3238.
- 42S. E. Denmark, Z. Wu, C. M. Crudden, H. Matsuhashi, J. Org. Chem. 1997, 62, 8288–8289.
- 43Y. Popowski, Y. Lu, G. W. Coates, W. B. Tolman, J. Am. Chem. Soc. 2022, 144, 8362–8370.
- 44Z. Jia, J. Jiang, X. Zhang, Y. Cui, Z. Chen, X. Pan, J. Wu, J. Am. Chem. Soc. 2021, 143, 4421–4432.
- 45J.-B. Zhu, E. M. Watson, J. Tang, E. Y.-X. Chen, Science 2018, 360, 398–403.
- 46M. Li, Y. Tao, J. Tang, Y. Wang, X. Zhang, Y. Tao, X. Wang, J. Am. Chem. Soc. 2019, 141, 281–289.
- 47Y. Zheng, S. Xu, C. Yu, P. Pan, Acc. Mater. Res. 2022, 3, 1309–1322.
- 48L. Feng, X. Bian, G. Li, X. Chen, Macromolecules 2021, 54, 10163–10176.
- 49F. Auriemma, C. De Rosa, M. R. Di Caprio, R. Di Girolamo, W. C. Ellis, G. W. Coates, Angew. Chem. Int. Ed. 2015, 54, 1215–1218.
- 50Y. Liu, W.-M. Ren, M. Wang, C. Liu, X.-B. Lu, Angew. Chem. Int. Ed. 2015, 54, 2241–2244.
- 51Y. Liu, M. Wang, W.-M. Ren, Y.-C. Xu, X.-B. Lu, Angew. Chem. Int. Ed. 2015, 54, 7042–7046.
- 52J. Li, B.-H. Ren, S.-Y. Chen, G.-H. He, Y. Liu, W.-M. Ren, H. Zhou, X.-B. Lu, ACS Catal. 2019, 9, 1915–1922.
- 53Z.-Q. Wan, J. M. Longo, L.-X. Liang, H.-Y. Chen, G.-J. Hou, S. Yang, W.-P. Zhang, G. W. Coates, X.-B. Lu, J. Am. Chem. Soc. 2019, 141, 14780–14787.