Aqueous Electroreduction of Nitric Oxide to Ammonia at Low Concentration via Vacancy Engineered FeOCl
Xiaoxi Guo
School of Materials Science and Engineering, Central South University, Changsha, 410083 Hunan, P. R. China
Hunan Joint International Research Center for Carbon Dioxide Resource Utilization, State Key Laboratory of Powder Metallurgy, School of Physics, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorPai Wang
Institute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu, 610054 Sichuan, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Tongwei Wu
Institute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu, 610054 Sichuan, P. R. China
Search for more papers by this authorZhiqiang Wang
Key Laboratory for Advanced Materials and Joint International Research Laboratory for Precision Chemistry and Molecular Engineering, Centre for Computational Chemistry and Research Institute of Industrial Catalysis, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 P. R. China
Search for more papers by this authorProf. Jiong Li
Shanghai Synchrotron Radiation Facility, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai, 201210 P. R. China
Search for more papers by this authorDr. Kang Liu
Hunan Joint International Research Center for Carbon Dioxide Resource Utilization, State Key Laboratory of Powder Metallurgy, School of Physics, Central South University, Changsha, 410083 Hunan, P. R. China
School of Metallurgy and Environment, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorProf. Junwei Fu
Hunan Joint International Research Center for Carbon Dioxide Resource Utilization, State Key Laboratory of Powder Metallurgy, School of Physics, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorProf. Min Liu
College of Nuclear Science and Technology, University of South China, Hengyang, 421001 Hunan, P. R. China
Search for more papers by this authorCorresponding Author
Dr. Jun Wu
School of Metallurgy and Environment, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorProf. Dr. Zhang Lin
School of Metallurgy and Environment, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorProf. Liyuan Chai
School of Metallurgy and Environment, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Zhenfeng Bian
MOE Key Laboratory of Resource Chemistry and Shanghai Key Laboratory of Rare Earth Functional Materials, Shanghai Normal University, Shanghai, 200234 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Hengfeng Li
School of Materials Science and Engineering, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Min Liu
Hunan Joint International Research Center for Carbon Dioxide Resource Utilization, State Key Laboratory of Powder Metallurgy, School of Physics, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorXiaoxi Guo
School of Materials Science and Engineering, Central South University, Changsha, 410083 Hunan, P. R. China
Hunan Joint International Research Center for Carbon Dioxide Resource Utilization, State Key Laboratory of Powder Metallurgy, School of Physics, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorPai Wang
Institute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu, 610054 Sichuan, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Tongwei Wu
Institute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu, 610054 Sichuan, P. R. China
Search for more papers by this authorZhiqiang Wang
Key Laboratory for Advanced Materials and Joint International Research Laboratory for Precision Chemistry and Molecular Engineering, Centre for Computational Chemistry and Research Institute of Industrial Catalysis, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 P. R. China
Search for more papers by this authorProf. Jiong Li
Shanghai Synchrotron Radiation Facility, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai, 201210 P. R. China
Search for more papers by this authorDr. Kang Liu
Hunan Joint International Research Center for Carbon Dioxide Resource Utilization, State Key Laboratory of Powder Metallurgy, School of Physics, Central South University, Changsha, 410083 Hunan, P. R. China
School of Metallurgy and Environment, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorProf. Junwei Fu
Hunan Joint International Research Center for Carbon Dioxide Resource Utilization, State Key Laboratory of Powder Metallurgy, School of Physics, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorProf. Min Liu
College of Nuclear Science and Technology, University of South China, Hengyang, 421001 Hunan, P. R. China
Search for more papers by this authorCorresponding Author
Dr. Jun Wu
School of Metallurgy and Environment, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorProf. Dr. Zhang Lin
School of Metallurgy and Environment, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorProf. Liyuan Chai
School of Metallurgy and Environment, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Zhenfeng Bian
MOE Key Laboratory of Resource Chemistry and Shanghai Key Laboratory of Rare Earth Functional Materials, Shanghai Normal University, Shanghai, 200234 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Hengfeng Li
School of Materials Science and Engineering, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Min Liu
Hunan Joint International Research Center for Carbon Dioxide Resource Utilization, State Key Laboratory of Powder Metallurgy, School of Physics, Central South University, Changsha, 410083 Hunan, P. R. China
Search for more papers by this authorGraphical Abstract
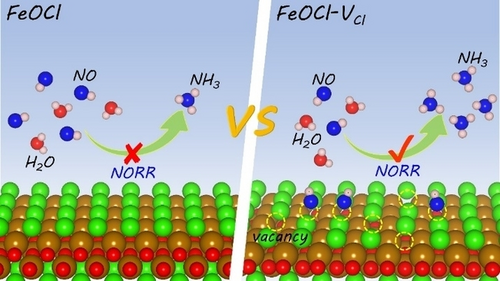
A Cl vacancy strategy results in a lower Fe oxidation state with sharp d-states characteristics to enhance the adsorption and activation of NO, which overcomes the sluggish kinetics of NORR and competitive HER over FeOCl-VCl, especially at low concentrations of NO, and improves the catalytic activity and selectivity towards NORR.
Abstract
Electroreduction of nitric oxide (NO) to NH3 (NORR) has gained extensive attention for the sake of low carbon emission and air pollutant treatment. Unfortunately, NORR is greatly hindered by its sluggish kinetics, especially under low concentrations of NO. Herein, we developed a chlorine (Cl) vacancy strategy to overcome this limitation over FeOCl nanosheets (FeOCl-VCl). Density functional theory (DFT) calculations revealed that the Cl vacancy resulted in defective Fe with sharp d-states characteristics in FeOCl-VCl to enhance the absorption and activation of NO. In situ X-ray absorption near-edge structure (XANES) and attenuated total reflection-infrared spectroscopy (ATR-IR) verified the lower average oxidation state of defective Fe to enhance the electron transfer for NO adsorption/activation and facilitate the generation of key NHO and NHx intermediates. As a result, the FeOCl-VCl exhibited superior NORR activities with the NH3 Faradaic efficiency up to 91.1 % while maintaining a high NH3 yield rate of 455.4 μg cm−2 h−1 under 1.0 vol % NO concentration, competitive with those of previously reported literatures under higher NO concentration. Further, the assembled Zn-NO battery utilizing FeOCl-VCl as cathode delivered a record peak power density of 6.2 mW cm−2, offering a new route for simultaneous NO removal, NH3 production, and energy supply.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202318792-sup-0001-misc_information.pdf2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Hwang, R. Rao, L. Giordano, K. Akkiraju, X. R. Wang, E. J. Crumlin, H. Bluhm, Y. Shao-Horn, Nat. Catal. 2021, 4, 663–673.
- 2L. Han, S. Cai, M. Gao, J. Hasegawa, P. Wang, J. Zhang, L. Shi, D. Zhang, Chem. Rev. 2019, 119, 10916–10976.
- 3T. Wu, H. Zhao, X. Zhu, Z. Xing, Q. Liu, T. Liu, S. Gao, S. Lu, G. Chen, A. M. Asiri, Y. Zhang, X. Sun, Adv. Mater. 2020, 32, 2000299.
- 4L. Ouyang, J. Liang, Y. Luo, D. Zheng, S. Sun, Q. Liu, M. S. Hamdy, X. Sun, B. Ying, Chin. J. Catal. 2023, 50, 6–44.
- 5Y. Ashida, K. Arashiba, K. Nakajima, Y. Nishibayashi, Nature 2019, 568, 536–540.
- 6Y. Huang, C. He, C. Cheng, S. Han, M. He, Y. Wang, N. Meng, B. Zhang, Q. Lu, Y. Yu, Nat. Commun. 2023, 14, 7368.
- 7S. L. Foster, S. I. P. Bakovic, R. D. Duda, S. Maheshwari, R. D. Milton, S. D. Minteer, M. J. Janik, J. N. Renner, L. F. Greenlee, Nat. Catal. 2018, 1, 490–500.
- 8G. Soloveichik, Nat. Catal. 2019, 2, 377–380.
- 9T. Wu, X. Zhu, Z. Xing, S. Mou, C. Li, Y. Qiao, Q. Liu, Y. Luo, X. Shi, Y. Zhang, X. Sun, Angew. Chem. Int. Ed. 2019, 58, 18449–1845.
- 10Y. Wang, H. Li, W. Zhou, X. Zhang, B. Zhang, Y. Yu, Angew. Chem. Int. Ed. 2022, 61, e202202604.
- 11S. Han, H. Li, T. Li, F. Chen, R. Yang, Y. Yu, B. Zhang, Nat. Catal. 2023, 6, 402–414.
- 12L. Zhang, J. Liang, Y. Wang, T. Mou, Y. Lin, L. Yue, T. Li, Q. Liu, Y. Luo, N. Li, B. Tang, Y. Liu, S. Gao, A. A. Alshehri, X. Guo, D. Ma, X. Sun, Angew. Chem. Int. Ed. 2021, 60, 25263–25268.
- 13J. Liang, P. Liu, Q. Li, T. Li, L. Yue, Y. Luo, Q. Liu, N. Li, B. Tang, A. A. Alshehri, I. Shakir, P. O. Agboola, C. Sun, X. Sun, Angew. Chem. Int. Ed. 2022, 61, e202202087.
- 14T. Wu, M. M. Melander, K. Honkala, ACS Catal. 2022, 12, 2505–2512.
- 15J. Liang, Q. Zhou, T. Mou, H. Chen, L. Yue, Y. Luo, Q. Liu, M. S. Hamdy, A. A. Alshehri, F. Gong, X. Sun, Nano Res. 2022, 15, 4008–4013.
- 16J. Liang, W. Hu, B. Song, T. Mou, L. Zhang, Y. Luo, Q. Liu, A. A. Alshehri, M. S. Hamdy, L. Yang, X. Sun, Inorg. Chem. Front. 2022, 9, 1366–1372.
- 17K. Chen, J. Wang, J. Kang, X. Lu, X. Zhao, K. Chu, Appl. Catal. B 2023, 324, 122241.
- 18Z. Li, Q. Zhou, J. Liang, L. Zhang, X. Fan, D. Zhao, Z. Cai, J. Li, D. Zheng, X. He, Y. Luo, Y. Wang, B. Ying, H. Yan, S. Sun, J. Zhang, A. A. Alshehri, F. Gong, Y. Zheng, X. Sun, Small 2023, 19, 2300291.
- 19J. Shao, H. Jing, P. Wei, X. Fu, L. Pang, Y. Song, K. Ye, M. Li, L. Jiang, J. Ma, R. Li, R. Si, Z. Peng, G. Wang, J. Xiao, Nat. Energy 2023, 8, 1273–1283.
- 20J. Long, S. Chen, Y. Zhang, C. Guo, X. Fu, D. Deng, J. Xiao, Angew. Chem. Int. Ed. 2020, 59, 9711–9718.
- 21S. Zhao, J. Liu, Z. Zhang, C. Zhu, G. Shi, J. Wu, C. Yang, Q. Wang, M. Chang, K. Liu, S. Li, L. Zhang, Chem 2023, 9, 3555–3572.
- 22G. Meng, M. Jin, T. Wei, Q. Liu, S. Zhang, X. Peng, J. Luo, X. Liu, Nano Res. 2022, 15, 8890–8896.
- 23J. Luo, M. Sun, C. L. Ritt, X. Liu, Y. Pei, J. C. Crittenden, M. Elimelech, Environ. Sci. Technol. 2019, 53, 2075–2085.
- 24W. Liu, L. Li, W. Shao, H. Wang, Y. Dong, M. Zuo, J. Liu, H. Zhang, B. Ye, X. Zhang, Y. Xie, Chem. Sci. 2023, 14, 1397–1402.
- 25N. Chubar, V. Gerda, M. Szlachta, G. Yablokova, Solid State Sci. 2021, 121, 106752.
- 26A. Atrei, B. Lesiak-Orlowska, J. Tóth, Appl. Surf. Sci. 2022, 602, 154366.
- 27R. Mehmood, W. Fan, X. Hu, J. Li, P. Liu, Y. Zhang, Z. Zhou, J. Wang, M. Liu, F. Zhang, J. Am. Chem. Soc. 2023, 145, 12206–12213.
- 28M. Sun, C. Chu, F. Geng, X. Lu, J. Qu, J. Crittenden, M. Elimelech, J. Kim, Environ. Sci. Technol. Lett. 2018, 5, 186–191.
- 29T. Park, G. C. Papaefthymiou, A. J. Viescas, A. R. Moodenbaugh, S. S. Wong, Nano Lett. 2007, 7, 766–772.
- 30S. K. S. Patel, S. Kurian, N. S. Gajbhiye, Mater. Res. Bull. 2013, 48, 655–660.
- 31M. T. Greiner, T. E. Jones, S. Beeg, L. Zwiener, M. Scherzer, F. Girgsdies, S. Piccinin, M. Armbrüster, A. Knop-Gericke, R. Schlögl, Nat. Chem. 2018, 10, 1008–1015.
- 32D. H. Kim, S. Ringe, H. Kim, S. Kim, B. Kim, G. Bae, H. Oh, F. Jaouen, W. Kim, H. Kim, C. H. Choi, Nat. Commun. 2021, 12, 1856.
- 33J. Fang, Q. Zheng, Y. Lou, K. Zhao, S. Hu, G. Li, O. Akdim, X. Huang, S. Sun, Nat. Commun. 2022, 13, 7899.
- 34Y. Li, C. Cheng, S. Han, Y. Huang, X. Du, B. Zhang, Y. Yu, ACS Energy Lett. 2022, 7, 1187–1194.
- 35J. Zhou, S. Han, R. Yang, T. Li, W. Li, Y. Wang, Y. Yu, B. Zhang, Angew. Chem. Int. Ed. 2023, 62, e202305184.