Enhancing the Crystallinity of Keto-enamine-Linked Covalent Organic Frameworks through an in situ Protection-Deprotection Strategy
Graphical Abstract
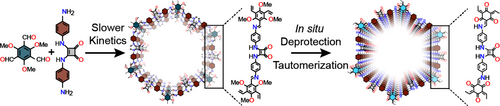
The protection of neighbouring groups during imine condensation reactions modulates the reactivity of building units in the synthesis of 2D covalent organic frameworks (COFs). The reduced reactivity of the protected aldehyde precursor decelerates the kinetics, making the reaction more reversible and improving the crystallinity of 2D COFs.
Abstract
β-Keto-enamine-linked 2D covalent organic frameworks (COFs) have emerged as highly robust materials, showing significant potential for practical applications. However, the exclusive reliance on 1,3,5-triformylphloroglucinol (Tp aldehyde) in the design of such COFs often results in the production of non-porous amorphous polymers when combined with certain amine building blocks. Attempts to adjust the crystallinity and porosity by a modulator approach are inefficient because Tp aldehyde readily forms stable β-keto-enamine-linked monomers/oligomers with various aromatic amines through an irreversible keto-enol tautomerization process. Our research employed a unique protection-deprotection strategy to enhance the crystallinity and porosity of β-keto-enamine-linked squaramide-based 2D COFs. Advanced solid-state NMR studies, including 1D 13C CPMAS, 1H fast MAS, 15N CPMAS, 2D 13C−1H correlation, 1H−1H DQ-SQ, and 14N−1H HMQC NMR were used to establish the atomic-level connectivity within the resultant COFs. The TpOMe-Sqm COFs synthesized utilizing this strategy have a surface area of 487 m2 g−1, significantly higher than similar COFs synthesized using Tp aldehyde. Furthermore, detailed time-dependent PXRD, solid-state 13C CPMAS NMR, and theoretical DFT studies shed more light on the crystallization and linkage conversion processes in these 2D COFs. Ultimately, we applied this protection-deprotection method to construct novel keto-enamine-linked highly porous organic polymers with a surface area of 1018 m2 g−1.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.