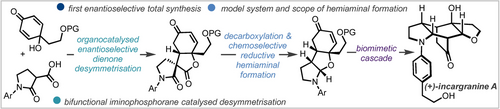
Enantioselective Total Synthesis of (+)-Incargranine A Enabled by Bifunctional Iminophosphorane and Iridium Catalysis
Graphical Abstract
The first enantioselective total synthesis of (+)-incargranine A has been completed in nine steps. An enantioselective, organocatalysed dienone desymmetrising Michael addition and an iridium-catalysed reductive cyclisation were developed to access a key hemiaminal intermediate that was used to initiate a biomimetic cascade towards the natural product.
Abstract
Herein we report the first enantioselective total synthesis of (+)-incargranine A, in nine steps. The total synthesis was enabled by an enantioselective intramolecular organocatalysed desymmetrising Michael addition of a malonamate ester to a linked dienone substrate that established pivotal stereocentres with excellent enantio- and complete diastereoselectivity. Furthermore, a key hemiaminal intermediate was accessed by developing an iridium-catalysed reductive cyclisation, and the scope of this transformation was explored to produce a range of bicyclic hemiaminal motifs. Once installed, the hemiaminal motif was used to initiate a biomimetic cascade to access the natural product directly in a single step.
The alkaloid incargranine A was first isolated from Incarvillea mairei var. grandiflora, commonly known as the Chinese Trumpet-Creeper, by Zhang and co-workers in 2009,1 featuring a [2,2,2]-bicyclooctane core with six contiguous stereocentres. Due to the near-zero measured specific rotation of natural incargranine A, there remains ambiguity as to its enantiopurity in nature. This dispute is exacerbated by the immense challenge in isolating incargranine A as a consequence of it making up just 0.002 ppm of the weight of the dried plant.1, 2 To date, there has been one previous total synthesis of racemic incargranine A carried out by Brown and Lawrence in 2019,2 in which they exploited a biomimetic approach to reach the target in an impressive four linear steps and 56 % overall yield. By completing this synthesis, they proposed a potential biosynthetic pathway to incargranine A from a hemiaminal-containing predicted natural product dia-millingtonine (Scheme 1A). This pathway consists of a retro-oxa-Mannich/oxa-Michael/Mannich cascade. In Lawrence's proposed biosynthesis the tricyclic core of dia-millingtonine is racemic, thus suggesting that natural incargranine A should also be racemic.
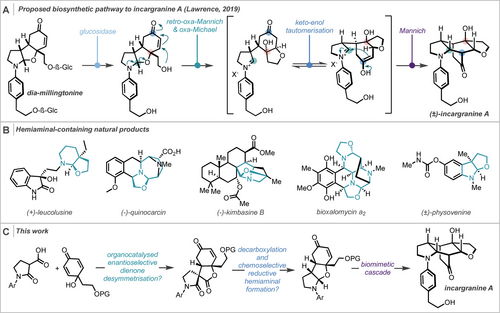
a) Biosynthetic cascade for incargranine A proposed by Lawrence; b) natural products containing bicyclic hemiaminals and c) overview of our planned synthesis of incargranine A.
With our interest in this natural product piqued by this debate, we believed that such an ambiguity lent itself perfectly to clarification by synthesis. An enantioselective total synthesis, providing access to a single enantiomer of the natural product, would reduce ambiguity surrounding the biosynthesis of incargranine A and, by extension, related natural products. Indeed, a successful resolution of this debate would elegantly highlight the power and utility of developing novel enantioselective C−C bond-forming methods as a tool to validate biosynthetic proposals and deconvolute biological processes. At the outset, we foresaw two main synthetic hurdles in completing an efficient enantioselective total synthesis: an effective and expeditious means to introduce enantioselectivity in the core; and, construction of the critical hemiaminal that would be key to trigger Lawrence's biomimetic cascade. As well as being crucial in our planned synthesis, cyclic hemiaminals are prevalent in many natural products (Scheme 1B), have been used as intermediates in total synthesis,3 and furthermore, select compounds containing this moiety have been shown to exhibit potent biological activity.4 Hence, robust and mild methods for their synthesis is desirable. Herein, we describe the first enantioselective total synthesis of incargranine A in nine steps, including a novel and highly enantioselective organocatalysed dienone desymmetrising Michael addition and a mild iridium catalysed reductive hemiaminal synthesis from alcohol-appended tertiary amides (Scheme 1C).
Our retrosynthetic analysis of incargranine A commenced by taking advantage of the proposed biosynthetic cascade to form hemiaminal I (Scheme 2). We envisaged reaching this key intermediate by the development of a challenging and bespoke chemoselective reductive cyclisation of tertiary amide II bearing a pendant tertiary alcohol to afford the hemiaminal, via the iminium ion formed in situ. Amide II could be accessed from spirocycle III by selective cleavage of the lactone and decarboxylation of the resultant carboxylate. Vital to our strategy was an enantio- and diastereoselective Michael addition to form spirocycle III from racemic dienone IV, constituting a late-stage dienone desymmetrisation. In order to introduce convergency, spirocycle III could be disconnected back to two simple fragments; disubstituted 2,5-cyclohexadienone A, and malonamic acid B. Therefore, our synthetic campaign could be divided into three main synthetic challenges: [1] the organocatalysed enantioselective dienone desymmetrisation; [2] the lactone cleavage and decarboxylation sequence to reach enone II; and, [3] the reductive hemiaminal formation.
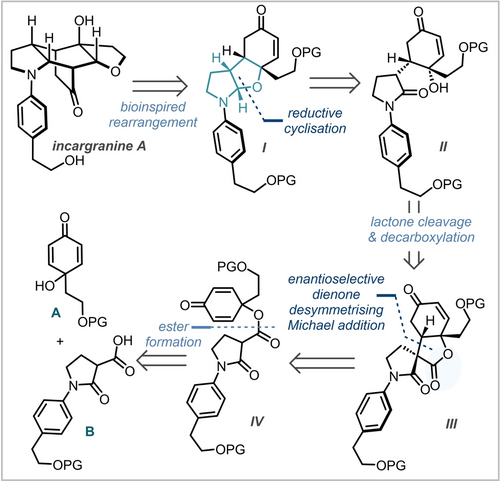
Retrosynthetic analysis of incargranine A.
To initiate investigations into the enantioselective dienone desymmetrisation, a concise route to Michael addition precursor IV was required. The synthesis of fragment B commenced with an Ullmann coupling between aryl bromide 1 and pyrrolidinone using catalytic copper iodide and 1,2-dimethylethylenediamine (DMEDA) which furnished 2 in near quantitative yield (Scheme 3).5 It should be noted that employment of Pd/Xantphos6 and copper iodide/dimethylethylamine based catalytic systems gave rise to poor conversion. Following 4-methoxy benzyl (PMB) protection of the primary alcohol, α-acylation of the lactam was carried out using sodium bis(trimethylsilyl)amide (NaHMDS) and diethyl carbonate to afford 3. Saponification of 3 under aqueous basic conditions, followed by acidification, furnished fragment B in 68 % yield over four steps. Fragment A was synthesised in two steps according to literature reports by primary alcohol tert-butyldimethylsilyl (TBS)-protection of tyrosol followed by hypervalent iodine oxidation of the resultant phenol.2 With both fragments in hand, attention then turned to their coupling and downstream advancement to the natural product. The union of fragments A and B was achieved under standard N,N′-dicyclohexylcarbodiimide (DCC) coupling conditions. Careful control of reaction temperature and time eliminated undesired, non-enantioselective Michael addition, to deliver coupled product 4 in 94 % yield. This expeditious route to dienone 4 enabled studies to begin on the pivotal organocatalysed enantioselective Michael addition.
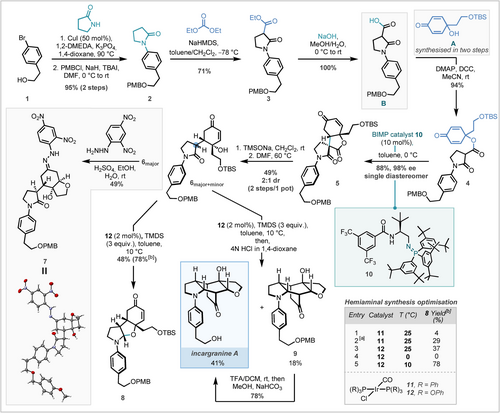
Total synthesis of incargranine A. [a] TMDS charged in two portions; [b] yields calculated by quantitative 1H NMR spectroscopy using 1,2,4,5-tetramethylbenzene as an internal standard. TBAI=tetrabutylammonium iodide, DMF=dimethylformamide, DMAP=4-(dimethylamino)pyridine.
Enantioselective desymmetrisation is an immensely powerful technique for the rapid generation of stereochemical complexity from achiral starting materials,7 and has been shown to be especially well-suited to the enantioselective synthesis of natural products.8 Furthermore, the prochiral 2,5-cyclohexadienone scaffold has been demonstrated to be a versatile scaffold for desymmetrisation9 with notable contributions in the field of enantioselective Michael additions by You,8d, 10 Harned,11 Deng,12 Shoji,13 and Gaunt.14 However, only one such cyclisation utilising a high pKa malonamate ester as a pro-nucleophile has been reported, which applied a cinchonidine-derived phase transfer catalyst, resulting in a low ee of 36 %.11 Inspiration to tackle this challenging step was drawn from previous work carried out in our group on the total syntheses of (−)-nakadomarin A that employed a bifunctional cinchona derived catalyst to carry out a diastereoselective intermolecular Michael addition of a malonamate ester pronucleophile with a highly reactive nitroolefin.15 Previous work has also demonstrated that bifunctional iminophosphorane (BIMP) organocatalysts can promote rapid and highly enantioselective Michael additions of pronucleophiles with pKa values comparable to that of malonamate esters.16 Hence, we hypothesised that a suitable BIMP catalyst could be identified, optimised and employed to execute this transformation. BIMP catalysts are a class of chiral organocatalysts discovered and developed by our group, comprising an H-bond donor moiety and a strongly basic iminophosphorane linked through a chiral backbone.17 Very conveniently, they are formed by an in situ Staudinger reaction of the corresponding azide and trivalent phosphine fragments, which allows rapid and modular diversification of the catalyst scaffold. We envisaged the deprotonation of the malonamate ester by the BIMP catalyst would result in a stereoselective Michael addition to enone 4, and, provided reversibility was avoided, high selectivity could be obtained.
Work was carried out into the enantioselective Michael addition by investigating a select range of BIMP catalysts, made in situ from their respective organoazide and triaryl phosphine components (Scheme 4). Pleasingly, first-generation thiourea azide (13 a) with electron-rich phosphine (14 a) yielded the desired product as a single diastereomer in 68 % yield and with significant but moderate enantioselectivity of 54 % ee (entry 1). Switching to a second-generation trans-substituted azide (13 b) provided a small increase in enantioselectivity, and a further enhancement was provided by squaramide (13 c), albeit with a small drop in yield. Finally, amide 13 d delivered the product in excellent yield and an impressively high enantioselectivity of 95 % ee (entry 4). Employing a more sterically-bulky phosphine (14 b) (entry 5) further elevated the enantioselectivity to 97 % ee. Carrying out the reaction in a range of other ethereal solvents showed no increase in ee, however toluene did provide an elevation in yield. Lowering the temperature resulted in an enhancement in enantioselectivity (entries 9 and 10), and 0 °C was found optimal for yield (entry 9). Pleasingly, up-scaling of this reaction to 1.3 mmol gave an uplift in yield (88 %) while preserving the very high enantioselectivity at 98 % ee (entry 11).
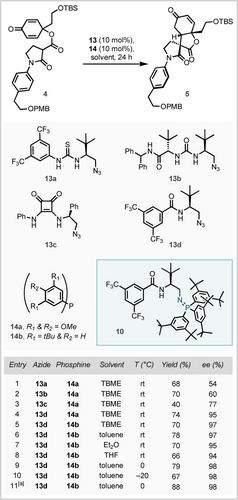
Optimisation of the BIMP-catalysed dienone desymmetrising Michael addition. All reactions carried out at 0.032 mmol scale and 0.16 M, yields calculated by quantitative 1H NMR spectroscopy using 1,2,4,5-tetramethylbenzene as an internal standard. TBME=tert-butyl methyl ether. [a] 1.3 mmol scale, 0.4 M and isolated yield.
We next sought to address the challenge of achieving chemoselective hydrolysis of the lactone moiety in 5 in preference to the neighbouring lactam. Standard conditions for lactone hydrolysis (potassium hydroxide in ethanol) showed little discrimination for the lactone functionality and hence were deemed unsuitable. Nicolaou had previously demonstrated trimethyltin hydroxide as a mild reagent to cleave esters selectively, however, employing these conditions resulted in no cleavage of the lactone.18 Employing caesium hydroxide for hydrolysis, to enable subsequent thermal or photochemical decarboxylation,19 only resulted in trace product formation and no reactivity could be obtained by transesterification approaches.20 Inspiration was eventually drawn from work by Deguin that showed remarkable reactivity of trimethylsilanoate (TMSONa) for lactone cleavage,21 although it's selectivity for lactones over fused lactams was previously unknown. It was found that the intermediate carboxylate showed low stability, but, to our delight, 6 was isolated as the major product following telescoped thermal decarboxylation.22 An investigation of this sequence, covering decarboxylation solvents, temperatures and additives (see Supporting Information for details), found optimal conditions that ultimately resulted in a yield of 49 % as an inconsequential 2 : 1 mixture of diastereomers. The absolute stereochemical configuration of the major diastereomer (6major) was determined by single crystal X-ray crystallographic analysis of 2,4-dinitrophenyl hydrazone derivative 7 (which was formed with concurrent TBS-deprotection and oxa-Michael addition of the resultant primary alcohol).23
The final remaining significant strategic transformation towards incargranine A was the key reductive cyclisation to form a hemiaminal from alcohol-tethered lactam 6 and the subsequent biomimetic cascade. Current methods to synthesise hemiaminals by employing an alcohol nucleophile typically require privileged precursors—for example α-amino nitriles3a, 24—or harsh conditions with lower functional group tolerance, such as strongly oxidising25 or reducing conditions.26 We foresaw forming the desired hemiaminal by a chemoselective iridium-catalysed reduction of an amide to produce a silylated hemiaminal,27 which upon treatment with acid would eliminate to the corresponding iminium ion, to which the pendant alcohol would then add to furnish a cyclic hemiaminal (Scheme 5). Due to its renowned chemoselectivity towards tertiary amides,28 we reasoned that Vaska's complex (IrCl(CO)(PPh3)2), in conjunction with silane reductant 1,1,3,3-tetramethyldisiloxane (TMDS), would be ideally suited for such a transformation. Aware of the potential chemoselectivity and reactivity challenges of this transformation at such a late-stage in the synthesis, we sought to validate this approach on a simpler yet structurally representative model series.
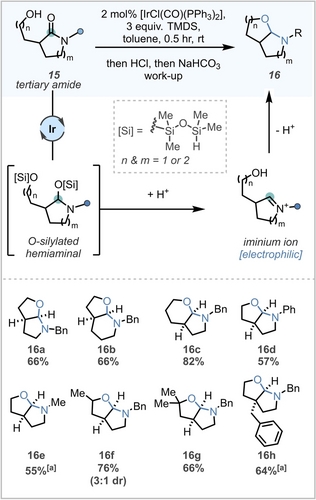
Scope of reductive hemiaminal cyclisation. All reactions carried out on 0.23 mmol scale and at 0.1 M. [a] Yield calculated by quantitative NMR spectroscopy using 1,2,4,5-tetramethylbenzene as an internal standard.
As a starting point, five-membered lactam 15 a was chosen as a model system as it would furnish the desired five-five bicyclic ring system. Initial studies were conducted as a series of 1H NMR experiments using 2 mol % of [IrCl(CO)(PPh3)2] with three equivalents of TMDS, one more than the typical two equivalents to account for concomitant silylation of the pendant alcohol. Reduction proceeded rapidly and quantitively at room temperature to deliver the corresponding silylated hemiaminal. Advantageously, upon treatment with dry 4 N HCl in 1,4-dioxane, it was observed that the resultant iminium was insoluble in the reaction solvent. Therefore, exploiting the iminium ion's water solubility, an acid/base extraction with aqueous 1 N HCl and saturated NaHCO3 was employed on the intermediate silylated hemiaminal to execute the desired cyclisation whilst minimising any unproductive side reactions. With optimal conditions established, the scope of this reaction was then explored. A range of five and six membered bicycles (16 a–c), as well those possessing different nitrogen substituents (16 a, d & e) were found to be well-tolerated. Crucially for the planned synthesis, both secondary (16 f) and tertiary (16 g) alcohols and further substitution on the backbone (16 h) was also tolerated. These structural features mapped well onto the backbone of many natural products, (Scheme 1B) and provided us with confidence to proceed with the proposed forward synthesis towards incargranine A.
The critical challenge in translating the model system reactivity to the total synthesis was to ensure chemoselectivity for reduction of the lactam over the enone moiety. Initially, employing Vaska's complex under the model system conditions resulted in poor selectivity (Scheme 3, table, entry 1). However, combining a phosphite derivative of Vaska's complex (12)29 and a lowered reaction temperature resulted in a highly selective reduction (entry 5). Fortuitously, in contrast to the model system, reduction of amide 6 directly produced hemiaminal product 8 in 78 % 1H NMR yield without the need for a work-up; this difference in outcome is likely linked to the increased steric bulk around the tertiary alcohol inhibiting silylation and/or a Thorpe Ingold effect promoting the direct cyclisation. Hemiaminal 8 could be isolated in 48 % yield, but to streamline the synthesis we sought to deprotect the TBS-group and trigger the retro-oxa-Mannich/oxa-Michael/Mannich cascade in a single step. Directly treating the reaction mixture with dry HCl in 1,4-dioxane achieved the desired reactivity with concurrent PMB-deprotection affording incargranine A in 41 % yield, in addition to PMB-protected incargranine A (9) in 18 % yield. Pleasingly 9 could be readily converted to incargranine A using trifluoroacetic acid (TFA). When each diastereomer of 6 was independently subjected to the reductive cyclisation conditions 9 was also isolated as the major product, highlighting that the reductive cyclisation reaction proceeds with epimerisation, presumably through the corresponding enamine intermediate. Chiral stationary phase HPLC analysis confirmed that our synthetic sample of incargranine A was highly enantioenriched with an ee of 98 % and the specific rotation of this sample was +13.6 (c 0.173 CHCl3). Although no authentic isolated sample of incargranine A was available for comparison, the reported specific rotation of the isolated incargranine A { +2 (c 0.175, CHCl3)} was sufficiently different for us to conclude that it is likely that natural incargranine A is produced as a racemate, in accordance with Lawrence's postulated biosynthesis of incargranine A from proposed natural product dia-millingtonine (Scheme 1A).
In summary, we have disclosed the first enantioselective synthesis of incargranine A in nine linear steps and 15 % overall yield. The success of the synthesis was enabled by introduction of two new synthetic transformations: a BIMP-catalysed enantioselective dienone desymmetrising Michael addition and an iridium-catalysed reductive hemiaminal synthesis from hydroxyethyl tethered lactams. The newly accessed hemiaminal was then used to trigger a biomimetic rearrangement to yield the natural product in a single step. The methodology explored in this synthesis has allowed insight into the biosynthesis of incargranine A and related natural products, supporting Lawrence's biosynthetic hypothesis and demonstrating the essential role that enantioselective natural product synthesis can play in exploring and understanding biological systems.
Acknowledgments
A.A.M.M. and B.D.A.S. are grateful to the Centre for Doctoral Training in Synthesis for Biology and Medicine for a studentship, generously supported by GlaxoSmithKline, MSD, Syngenta, and Vertex. A.A.M.M. thanks the Oxford-Radcliffe Scholarship and is grateful for to the Royal Commission of the Exhibition of 1851 for an Industrial Fellowship. P.B. is grateful for funding from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No. 892540. B.D.A.S. thanks the Oxford-Leon E & Iris L Beghian Scholarship for funding. The authors are grateful to Yaseen A. Almehmadi, Charmaine Y. X. Poh and J. Andrew P. Maitland, as well as other group members for catalyst synthesis and proof reading this manuscript. Timothy A. Davidson (University of Oxford) is thanked for X-ray data collection and structure determination and Dr. Kirsten E. Christensen (Oxford Chemical Crystallography Service) for X-ray mentorship.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.