Boosted Mg−CO2 Batteries by Amine-Mediated CO2 Capture Chemistry and Mg2+-Conducting Solid-electrolyte Interphases
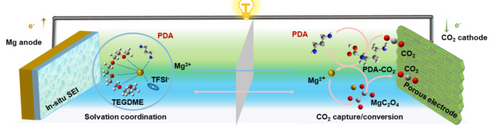
Graphical Abstract
A non-aqueous Mg−CO2 battery with high reversibility and enhanced kinetics is utilized using an amine molecular mediator that tunes the CO2 reduction pathways as well as the Mg anode. It not only modifies the reactant state of CO2 and Mg2+ to promote CO2 cathodic reaction kinetics but also facilitates Mg2+-conducting interphase formation to enable the reversible cycling of Mg anode.
Abstract
Mg−CO2 battery has been considered as an ideal system for energy conversion and CO2 fixation. However, its practical application is significantly limited by the poor reversibility and sluggish kinetics of CO2 cathode and Mg anode. Here, a new amine mediated chemistry strategy is proposed to realize a highly reversible and high-rate Mg−CO2 battery in conventional electrolyte. Judiciously combined experimental characterization and theoretical computation unveiled that the introduced amine could simultaneously modify the reactant state of CO2 and Mg2+ to accelerate CO2 cathodic reactions on the thermodynamic-kinetic levels and facilitate the formation of Mg2+-conductive solid-electrolyte interphase (SEI) to enable highly reversible Mg anode. As a result, the Mg−CO2 battery exhibits boosted stable cyclability (70 cycles, more than 400 h at 200 mA g−1) and high-rate capability (from 100 to 2000 mA g−1 with 1.5 V overpotential) even at −15 °C. This work opens a newly promising avenue for advanced metal-CO2 batteries.
Introduction
Due to the increasingly severe environmental pollution and global warming, clean fixation, conversion and utilization of the greenhouse gas-CO2 have become one of the most prevalent and pressing research topics for achieving sustainable development.1 Among all the proposed systems to reduce CO2 emission, rechargeable metal-CO2 batteries have been considered as a promising strategy for effective CO2 reduction with few pollutions, which would, in turn, provide renewable energy for further fixation of CO2.2 According to the type of anodes, metal-CO2 batteries are mainly divided into Li−CO2, Na−CO2, K−CO2, Mg−CO2, Zn−CO2, and Al−CO2 systems.3 However, the high activity, high cost or dendrite nature may hinder the practical development the Li/Na/K−CO2 batteries.4 Zn−CO2 and Al−CO2 are relatively low cost and low reactivity, but always suffer from the extremely low discharge voltage or high overpotential due to the inherently higher electric density and electrode potential of Zn and Al metal anodes (−0.76 V vs. Standard Hydrogen Electrode (SHE) and −1.66 V vs. SHE, respectively).5-7 With the safety and economy in mind, Mg metal has been considered as an ideal anode for metal-CO2 battery system due to its unique features, i.e. relatively low-reduction potential (−2.37 V vs. SHE), high-volumetric capacity (3832 mAh cm−3), dendrite-free nature at moderate current density and abundant reserves (2.9 %).8, 9 Matched with nonaqueous and cost-effective conventional electrolytes, Mg−CO2 battery exhibits tremendous promise for carbon utilization for efficient energy storage and conversion.
However, the nonaqueous rechargeable Mg−CO2 batteries are still infant with practical problems of high polarization, limited cycle current and/or delivery capacity. It would, actually, come down to two impediments: 1) Poor reversibility. Carbonate, MgCO3 in Mg−CO2 system specifically, considered as the final discharge product for the CO2 reduction reaction (CO2RR), is insulating and almost indecomposable.10, 11 It will thus accumulate on cathode, increasing the interfacial impedance and bruising the CO2 evolution reaction (CO2ER) upon recharge. In addition, the passivation of Mg anode in conventional electrolytes of course exacerbates the irreversibility further, leading to irreversible Mg−CO2 battery with a large overpotential and poor cycling performance. 2) Sluggish kinetics. Besides the acknowledged barriers for the cathodes including difficult de-solvation at cathode-electrolyte interface and sluggish Mg2+ diffusion within cathodes,12 the kinetics of CO2RR and CO2ER at cathode are also hindered by the poor compatibility of ternary-phase interface, difficult breakage of C=O bonds and incomplete decomposition of discharge products.13 As a result, Mg−CO2 batteries often have the problems of deficient cycle current, limited capacity and fast capacity decay. Significant efforts like structural cathodes,14 designed catalysts,15, 16 and reaction mediators,17, 18 etc., have been devoted to improve interfacial contacts, drive CO2 reduction or facilitate discharge product decomposition. However, due to the intrinsic properties of strong polarization and multi-electron transfer in Mg−CO2 system, these reported strategies can hardly care the cathodic reversibility and kinetics simultaneously, together with the anode reversibility. Liquid amines, on the one hand, have been confirmed for avoiding passivation on Mg anode via the regulation of solvation structure and the anode interphase.19, 20 On the other hand, this motif have also been studied abundantly for activating electrochemical CO2 conversion via chemical capturing.21, 22 Therefore, it is expected to adopt liquid amines to solve the notorious problems in Mg−CO2 batteries.
Here we propose a “kill two birds with one stone” strategy to realize a highly reversible and high-rate Mg−CO2 battery in conventional electrolyte via liquid 1, 3-propylene amine (PDA) medium chemistry approach (Figure 1a). By chemisorbing CO2 and tailoring Mg2+ solvation coordination, the introduction of PDA can benefit for both cathode and anode. For cathodic reaction, PDA could improve the compatibility of multiphase interface, facilitate fast Mg2+ de-solvation and diffusion, reduce the barrier of CO2RR, render the formation of decomposable discharge product−MgC2O4, and in turn, enhance the kinetics of CO2ER.13, 23 For anodic reaction, PDA induces the in situ formation of Mg2+-conductive solid electrolyte interphase (SEI), enabling highly reversible Mg plating/stripping. Therefore, with the promotion of PDA medium, a highly reversible Mg−CO2 battery with lifespan of over 400 h at 200 mA g−1 and superior rate performance from 100 to 2000 mA g−1 could be obtained in 0.25 M Mg(TFSI)2-TEGDME electrolyte. Importantly, the system could also operate normally under low temperature of 0 °C or −15 °C, demonstrating the high CO2 utilization ability and potential prospect of Mg−CO2 batteries. The demonstrated amine-mediated chemistry strategy for promoted CO2 capture and conversion provides a new avenue for developing high-performance metal-CO2 batteries.
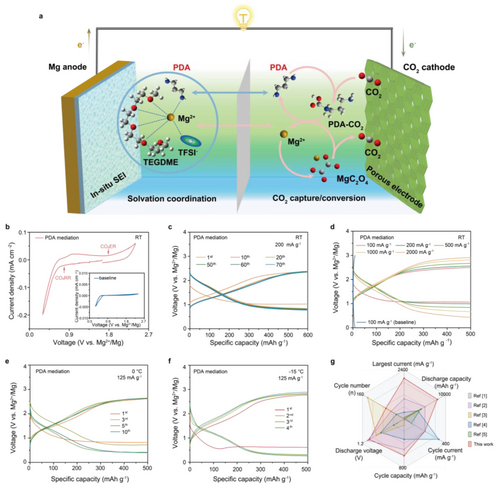
Electrochemical performance of Mg-CO2 battery promoted by PDA chemistry. a) Schematic diagram of a Mg−CO2 battery with PDA additive as bifunctional medium. b) CV curves of Mg−CO2 batteries with PDA additive from 0.3 to 2.4 V at a scan rate of 0.2 mV s−1. Inset: corresponding curve in baseline electrolyte. Selected discharge/charge cycles of Mg−CO2 batteries with PDA mediation c) at 200 mA g−1 with a capacity of 600 mAh g−1, e) at 125 mA g−1 with a capacity of 500 mAh g−1 under 0 °C and f) −15 °C. d) Rate performance under different current densities of Mg−CO2 battery with PDA mediation, and discharge/charge cycle of Mg−CO2 batteries without PDA mediation at 100 mA g−1. g) Electrochemical performance comparison of multivalent metal-CO2 batteries in nonaqueous electrolyte.
Results and Discussion
The Mg−CO2 battery was composed of Mg metal as anode and nitrogen-doped carbon nanotubes (N-CNTs) as catalytic cathode. The actual assembled cell and the corresponding schematic diagram were displayed in Figure S1. 0.25 M Mg(TFSI)2 in TEGDME (G4) was used as the baseline electrolyte for its chemical stability and commercial accessibility. 10 wt % PDA added in electrolyte was chosen as the optimal concentration according to the polarization and cyclic voltammetry (CV) tests for Mg anode (Figure S2). In this case, the CV curve of Mg−CO2 system with PDA electrolyte exhibits remarkable CO2RR (0.8 V) and CO2ER (1.8 V) peaks with large current response, demonstrating the efficient CO2 conversion involves in the electrochemical reactions. In contrast, without PDA, Mg−CO2 battery operates irreversibly, as evidenced by the extremely low current response shown in inset CV curve (Figure 1b). Due to the reversible anode and promoted cathode reactions, the PDA-mediated Mg−CO2 battery could maintain stable cycling for 70 cycles at 200 mA g−1 with a specific capacity of 600 mAh g−1 (Figure 1c), and display a lifespan over 400 h with overpotential from 1.3 to 1.5 V (Figure S3). Increasing the cycling capacity to 1000 mAh g−1, the battery could still operate for 40 cycles at 200 mA g−1 without pronounced overpotential build-up (Figure S4). Moreover, superior rate performance could be also achieved with stable voltage plateaus at current densities from 100 to 2000 mA g−1. In sharp contrast, Mg−CO2 battery without PDA fails with extremely high overpotential and rapid capacity decay (Figure 1d, Figure S5). The promoted kinetics by PDA mediation can be further evidenced by the stable cycling of the batteries even under low temperature of 0 °C and −15 °C (Figure 1e–f, Figure S6), as well as the deep utilization of CO2 which is reflected through the high discharge capacity of 4 mAh when Mg−CO2 with PDA behaves as primary battery (Figure S7). The above batteries could also output a stable voltage to power a light-emitting diode (LED), further manifesting its practical feasibility. Considering both the long-term cycling and rate performance, the rechargeable Mg−CO2 battery with PDA in our work have reached the highest level compared previous related literatures (Figure 1g and Table S5).
To understand the excellent performance of PDA-mediated Mg−CO2 cycling, the cathodic reaction pathway behind the CO2 conversion reaction is identified. After discharge process, as evidenced by scanning electron microscopy (SEM), spherical particles with only ≈100 nm in size were deposited on the interior and surface of porous N-CNTs mats cathodes, with Mg, C and O elements distributed evenly. The small particles, with short charge transfer pathways and exposed large surface areas, could be almost wholly reversible during subsequent recharge processes (Figure 2a, Figure S8–9). X-ray diffraction patterns (XRD) determined the main discharge product species is MgC2O4 (PDF#26–1222), whose peaks disappeared during charge process, corresponding well with the recovered cathode in SEM images (Figure 2b). Raman spectra also support the results. As depicted in Figure 2c, a new peak assigned to C2O42− (1500 cm−1) appears between the D band and G band after discharge.24 Meanwhile, there is no signal of amorphous carbon on the discharged carbon-free Ni@Ru cathode, suggesting that MgC2O4 is the only discharge product. The reversibility of MgC2O4 after recharging could be embodied through the observed weakened peaks (Figure 2d). The similar evolution of C=O, C−O, Mg−O vibration bands belonging to MgC2O4 could be also observed in Fourier transform infrared (FTIR) spectra (Figure 2e).25 Furthermore, the X-Ray photoelectron spectroscopy (XPS) peaks assigned to C2O42− (289.1 eV in C 1s, 532.9 eV in O 1s) along with divalent Mg2+ (51.3 eV in Mg 2p) also confirm the formation and decomposition of MgC2O4 product during discharging and charging (Figure 2f, g and Figure S10).26
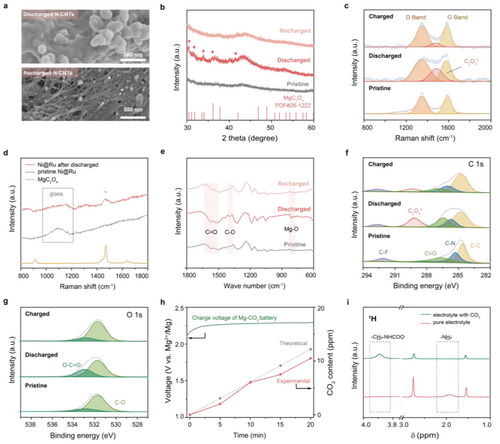
CO2 capture chemistry and discharge products analysis. a) SEM images of cathodes after discharge and recharge process. b) XRD patterns and c) Raman spectra of cathodes at pristine, discharged and charged states, respectively. d) Raman spectra of the pristine and discharged Ni@Ru cathode. e) FTIR spectra and f, g) XPS spectra of C 1s, O 1s of cathodes at pristine, discharged and charged states, respectively. h) Online CO2-evolution test and charge voltage of the pre-discharged Mg−CO2 battery at 100 mA g−1 with PDA mediation. i) 1H NMR spectra of 0.25 M Mg(TFSI)2-G4/10 %PDA electrolyte before/after CO2 treatment.
Taking all the results together, the cathodic pathway in Mg−CO2 battery with PDA mediation is reversible and can be proposed as Mg+CO2↔MgC2O4. Online CO2 evolution test was conducted to further verify this reaction (Figure 2h). During the recharge process, the voltage of the pre-discharged Mg−CO2 battery remains at around 2.3 V (green line), the recorded CO2 evolution amount, calculated by the formula CO2 content (ppm)=n(CO2)/[n(CO2)+n(Ar)], accumulates with time (red line) and is close to the theoretical value (grey dots). The results suggest that, instead of MgCO3 with wide band gap and high thermodynamic stability requiring a high decomposition energy, more conductive and easily decomposed MgC2O4 contributes to the highly reversible cycling of CO2RR and CO2ER. PDA serves as the key to the unusual reaction pathway. Whether in the bulk phase of liquid electrolyte or at the interphase of the N-CNT substrate, the strong interaction between PDA and CO2 would always exist (Figure S11–13, Table S6–8), under which the C=O bond length could be stretched to a large extent and, the inert linear O=C=O bond configuration would twist to nearly ≈122°, closer to O=C=O conformation in MgC2O4. Tracing back to its properties, we speculated that PDA, a kind of amine sorbents capturing CO2 efficiently,21, 22 modified the reactant state of CO2 and tuned the CO2RR process through a conversion reaction (NH2-(CH2)3−NH2+CO2→NH2-(CH2)2−CH2−NHCOOH), which was proved by proton nuclear magnetic resonance (NMR) spectra (Figure 2i). Upon introduction of CO2 in electrolyte with PDA, the 1H NMR resonance of −NH2, initial observed at 1.9 ppm, disappeared and instead a new peak occurred at 3.9 ppm, representing the formation of carbamic group.21, 27 The formed −CH2−NHCOO- in liquid electrolyte would be capable of subsequent electrochemical reactions through the easier N−C bond cleavage in place of gaseous CO2 with the resultant products (MgC2O4 mentioned above).
The MgC2O4 consisting of only CO2-derived species also indicate that PDA would meanwhile recover and return to electrolyte for repeatedly CO2 uptake. This CO2 capturing chemistry has further been confirmed through the discharging behaviors in pure Ar of assembled batteries after initially stood in CO2 for 12 h. Bi−Mg anodes rather than pure Mg were used to exclude the influence of passivation issue in baseline electrolyte.28 With the assistance of PDA in electrolyte, the Mg−CO2 discharge reaction happened with voltage about 0.75 V, indicating that the absorbed CO2 to −CH2−NHCOO- takes part in the electrochemical reaction as active species. While without PDA, the voltage of battery is lower than 0.4 V, which is mainly due to the capacitive behavior of N-CNT cathode without the CO2 participation (Figure S14).
Density functional theory (DFT) calculations were adopted to further provide an in-depth understanding of CO2RR selectivity with PDA involved and identify the origin of optimized cathodic reaction behaviors. Based on previous reports,10, 13, 29, 30 it is proposed that MgCO3 nucleation in baseline system undergoes pathway I, while MgC2O4 nucleation in PDA mediated system is possibly initiated by pathway II and furthermore, hypothetic MgCO3 nucleation with PDA mediation has been listed in pathway II′ for comparison (Figure S15).
We here discuss the reaction thermodynamics through calculated Gibbs free energy change (▵G) between different intermediates (Figure 3a). In all the pathways, carbonate/oxalate generations accompanied by electron transfer processes are always uphill in the energetic profiles, indicating it proceeds rate-limiting steps. The energy barriers, ▵G, of this controlling step for MgCO3 nucleation in pathway I, MgC2O4 nucleation in pathway II, MgCO3 nucleation in pathway II′ are 1.394, 0.748 and 1.386 eV, respectively. To fully overcome the energy barrier, the corresponding applied voltages are calculated to 1.394 V, 0.748 V and 1.386 V in theory (Figure S16). That suggests the electro-reduction of active reactant −CH2−NHCOO- in PDA tailoring system can be much easier than the sluggish free CO2 in baseline and more importantly, instead of almost indecomposable MgCO3, more conductive and readily decomposed MgC2O4 has been proved to be the thermodynamically favored discharge product in PDA involved reaction pathway.
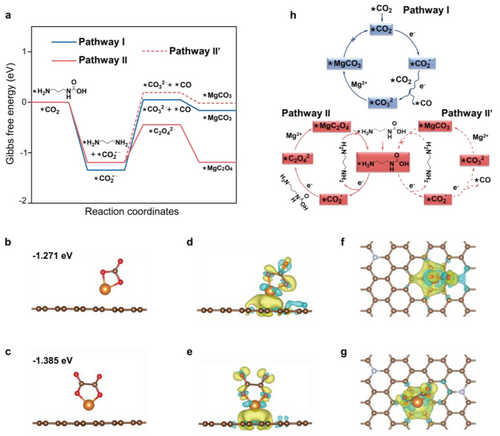
Mechanism of CO2 conversion chemistry. a) Gibbs free energy change between different intermediates for MgCO3 nucleation in baseline system, MgC2O4 and MgCO3 nucleation in PDA mediated system. b) The adsorption of MgCO3 and c) MgC2O4 on N-CNT. d, f) The differential charge density upon adsorption of MgCO3 and e, g) MgC2O4, the yellow and cyan isosurfaces represent the gain and loss of electron density, respectively from d, e) the side and f, g) top-down views. h) Schemes of reaction process of pathway I, pathway II and pathway II′.
Adsorption interactions between MgCO3/MgC2O4 and N-CNT substrate were further analyzed. Considering the different adsorption modalities, the optimized configurations and energies have been obtained (Figure 3b, c and Figure S17). Obviously, the adsorption of MgC2O4 on N-CNT is more preferable with larger negative energy (−1.385 eV) than that of MgCO3 (−1.271 eV). Electron-withdrawing N in N-CNT can stimulate its neighboring C atoms to be active adsorption sites,31, 32 and consistently, the adsorption interactions here mainly derive from the electron donation of N and adjacent C atoms to Mg atoms in adsorbates ( Figure 3d–g). The stronger surface affinity may hinder the MgC2O4 molecules aggregation and relieve its subsequent disproportionation reaction.13 The high energy barrier for MgC2O4 splitting to MgCO3 on N-CNT substrate further verify the relative stability of MgC2O4 (Figure S18, 19),11, 33 which supports the favorability of MgC2O4 as the discharge product, facilitating the reversibility of entire Mg−CO2 reaction cycle in PDA mediated system (Figure 3h).
Thermodynamic dominance endows the cathode with higher reversibility, while kinetic factors determine the actual reaction rate. In this regard, by pre-incorporating CO2 into electrolyte with the PDA adduct, the gas-liquid-solid ternary phase reaction at cathode side can be transformed into liquid-solid two phase. The face-to-face interface overcomes the incompatibility and large resistance in ternary phase reactions therefore,18, 34 significantly enhancing the kinetics of CO2ER and CO2RR.
As analyzed above, PDA mainly acts as a catalytic mediation for cathodic reaction and remains in electrolyte solution. Therefore, Mg2+ solvation environment, may get changed due to the introduced PDA and this will affect the reaction kinetics.
Electrostatic potential (ESP) mapping implies the electronegativity of nitrogen atoms in PDA is a bit stronger than that of oxygen atoms in G4 and thus, PDA could theoretically partially displace G4 to coordinate with electron-withdrawing Mg2+ and soften the restriction between G4 and Mg2+ (Figure 4a). The interaction between residual PDA and pristine electrolyte have been probed by NMR analysis of various solution compositions. In contrast with the pure G4 (I), the 13C signal of methyl carbon (−CH3) in G4 shifts down-field in the presence of Mg(TFSI)2 (II), revealing the formation of stable Mg2+−G4 coordination solvation structure.35, 36 When incremental amounts of PDA were introduced (III−V), the relative signals move to up-field in reverse, and gradually close to pure solvent state (Figure 4b). The correlative 1H spectra also display the similar tendency (Figure S20), indicating that PDA weakens the coordination between Mg2+ and G4 solvent molecule. The 1H NMR resonances belonging to PDA likewise illustrate the interaction (Figure 4c). Broader 1H signal with down-field chemical shift can be observed in 0.25 M Mg(TFSI)2-PDA (VII) compared to pure PDA (VI), implying PDA is prone to coordinate with Mg2+. For the electrolyte with PDA (III−V), the changes in 1H NMR resonances of −NH2 protons are more pronounced, that is, the electron density and shielding effect of nitrogen atoms in PDA decrease,27 in which the coordination with Mg2+ mainly occurs and therefore, the original Mg2+ solvation coordination has been changed. Raman spectra also reflect the solvation sheath structure change with PDA additive in electrolytes (Figure S21). The broad band around 870 cm−1 is associated with C−O stretching and CH2 rocking modes in free G4 solvent (I), while the newly appeared bands at 560 and 895 cm−1 with Mg(TFSI)2 existence are attributed to Mg2+−G4 complex (II).37, 38 With the increasing amount of PDA in electrolyte, the two peaks reveal red-shift or even disappearance (gray line b and c), suggesting the weaker interaction between Mg2+ and G4.39 The enhanced coordination of Mg2+ with PDA could be validated through blue-shift of NH2 bands at about 420 and 940 cm−1 (gray line a and d).40
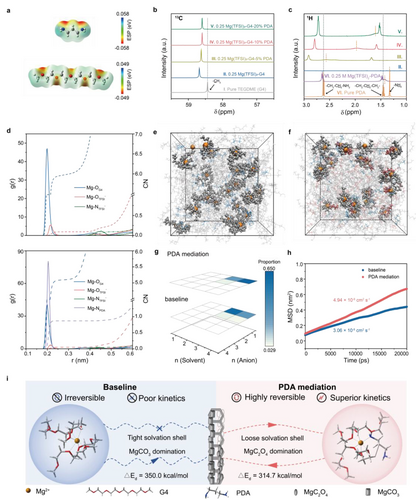
Solvation coordination of Mg2+ with PDA modification. a) Electrostatic potential of G4 and PDA. b) 13C NMR spectra of pure G4 and electrolyte with/without PDA. c) 1H NMR spectra of pure PDA, Mg(TFSI)2-PDA and electrolyte with/without PDA. d) RDF and CN of TFSI−, G4 and PDA in baseline and PDA mediated electrolytes. e) Snapshots of baseline electrolyte and f) PDA mediated electrolyte captured from MD trajectories. Mg2+, TFSI−, G4 and PDA were colored in brown, blue, gray and red, respectively, with the Mg2+ solvation structures highlighted using the ball-and-stick model. g) The distribution of Mg2+ solvation clusters in different electrolytes. h) Mg2+ self-diffusion coefficients from MD simulations. i) Schematic illustration of Mg2+-solvation structure and corresponding features.
Molecular dynamics (MD) simulations were performed to explore the solvation structures in detail. The radial distribution functions (RDF) and coordination numbers (CN) of anions or solvents were calculated. As shown in Figure 4d, baseline and PDA mediated electrolytes both display negligible anions coordination, evidenced by the low CN values of Mg−OTFSI− and Mg−NTFSI−. The RDF of Mg−OG4 with CN of 6.0 around indicates the aggregated Mg−G4 clusters are the main species in baseline system. After PDA introduced, there is a strong interaction between NPDA and Mg cations, so the CN of Mg−OG4 reduces to 5 along with the emerged Mg−NPDA coordination. Thus, in agreement with NMR and Raman results, the main role of PDA in electrolyte is to squeeze G4 partially out of the solvation sheath. Corresponding Mg-oriented aggregates can be observed in the MD snapshots (Figure 4e, f), whose occurring proportions have been counted (Figure 4g, Table S9). It turns out that both electrolytes exhibit the largest number of solvent-dominated solvates, namely [Mg(G4)2]2+ and [Mg(G4)2(PDA)1]2+ with or without PDA participation. Although the number of involved G4 has not changed, the optimized solvation structures show that PDA breaks its tight chelating effects and soften the coordination interaction between Mg2+ and surrounding solvents (Figure S22). Consistent with it, the Mg2+ diffusion coefficient fitted by MD, conductivity and Mg2+ transfer number have all been increased with PDA mediation, specially, from 3.06×10−8 to 4.94×10−8 cm2 s−1, 1.46 to 2.08 mS cm−1 and 0.36 to 0.45, respectively (Figure 4h, Figure S23–24). Beyond that, the Mg2+ dissociation energy ( ), which could quantitatively evaluate the de-solvation energy barrier of Mg2+, decreases from 350.0 kcal mol−1 to 314.7 kcal mol−1 using DFT calculation and, the similar tendency can be also verified by MD simulation (Table S10–11, Figure S25), suggesting that Mg2+ is more kinetically favorable to dissociate in PDA-mediated solvation shell compared to baseline. For the completion of the whole Mg−CO2 reaction, not only the state of CO2 that been modified by PDA affects the reaction kinetics, as another reactant, the state of Mg2+ also plays an important role. In other word, before accepting the electrons, Mg2+ would be dissociated from the cluster containing many solvents or anions. Thus, the regulated loose Mg2+ solvation shell is anticipated to facilitate fast charge transfer at cathodic interface and thus improve Mg−CO2 performances. Combining with the MgC2O4 nucleation domination by the modification of CO2, the high reversibility and superior kinetics of CO2 redox can be largely guaranteed (Figure 4i).
In addition to cathode, the electrochemical behaviors of anode also affect the whole Mg−CO2 battery performance. As listed in Figure S2, PDA with appropriate amount alleviates anode passivation, enabling long-term reversible plating/stripping. Solvation structures mentioned above also occupy a dominant place for the improved anode. Bulk tailored Mg2+ ion transport surely avails the reduced overpotentials, yet the anode-electrolyte interphase induced by modified solvation shell also accelerates the charge transfer kinetics.
Depth XPS with Ar+ etching was performed to unveil the evolution of anode interphase. For cycled Mg in PDA-containing electrolyte (Figure 5a–c), a emerged peak at 400.4 eV of N−H group, accompanied by a stronger peak of C−N group at 399.8 eV in N1s, are mainly derived from partial PDA adsorption or reduction on Mg anode.20, 41 The preferential interactions between Mg and PDA mitigate the decomposition of TFSI− on the Mg surface, as evidenced by the lower ratio of notorious passive and Mg2+conduct-inhibiting components (MgF2, MgNx, MgO, MgCO3 and MgSOx)42, 43 than that originated from cycled Mg in baseline electrolyte in N1s, F1s, Mg2p, O1s, C1s and S2p spectra (Figure S26, 27). As a result, the interphase arising from decomposed PDA is rich in organic species (C−C, C−O, C−F, C=O, C−N, N−H) that will be easily etched out and thus, the Mg0 signal increases evidently after 600s Ar+ sputtering. Similar with Ca2+-conductive interphase,44 the organic-enhanced thin layer would facilitate Mg2+ diffusion, enabling reversible Mg plating/stripping. In sharp contrast, Mg anode undergoes passive interface mainly imbued with the inorganic TFSI− decomposition products (Figure 5d–e). Beyond the intrinsic unsurmountable barrier for Mg2+ diffusion, anion-derived passivation layer is even thicker proved by the weak exposure of Mg0 signal within the same etching time, which would elongate the Mg2+ migration pathway and exacerbate the anodic kinetics. Further conducted SEM characterizations are consistent with all the results obtained from XPS measurements (Figure S28). Basically, the interphase constructed in the electrolyte mediated by PDA is dominated by organic species, which is more conducive to Mg2+ transport than the much thicker interphase containing mainly inorganic passive components formed in PDA-free electrolyte, thus ensuring the reversibility of Mg anode for Mg−CO2 battery (Figure 5g–h). It has also been illustrated in CV curves (Figure 5i). Compared to negligible current response for the Mg||SS in pristine electrolyte, an additional reduction peak can be observed in the initial few cycles for that in electrolyte with PDA, apart from a pair of reduction/oxidation peaks related to Mg metal plating/stripping process, suggesting the decomposition of PDA that induces the in situ formation of Mg2+ conductive SEI layer on Mg anode. After 10 cycles, the responsive currents gradually keep stable along with only plating/stripping peaks, indicating the complete formation of stable SEI and thus the efficient Mg plating/stripping.
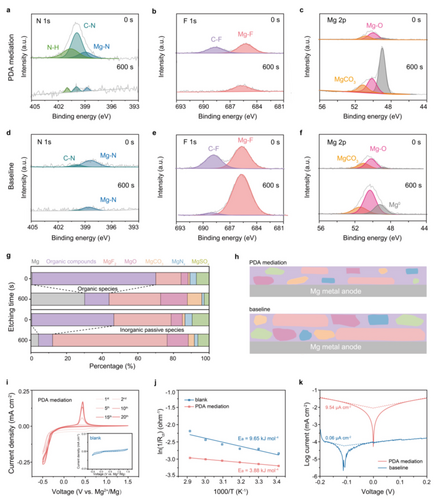
PDA mediated electrolyte for reversible Mg anode interphase. a–c) XPS spectra for N 1s, F 1s and Mg 2p of cycled Mg anodes in electrolyte with PDA and d–f) without PDA before and after 600s Ar+ sputtering. g) The relative proportions of decomposition species and h) corresponding schematic illustrations of cycled Mg anodes in electrolyte with or without PDA. i) CV curves of Mg||SS asymmetric batteries at a scan rate of 5 mV s−1 from −0.5 to 1.5 V at different cycles with PDA additive. Inset: corresponding CV curve with blank electrolyte. j) Arrhenius curves and activation energy in two different electrolytes. k) Tafel plots of symmetric cells with or without PDA.
Benefiting from the synergistic effects of favorable electrolyte solvation structure and in situ formed Mg2+-conducting SEI, the significantly enhanced charge transfer kinetics of anode can be realized. Specially, the calculated activation energy (Ea) has increased by 149 % (from 3.88 kJ mol−1 to 9.65 kJ mol−1) with PDA mediation (Figure 5j and Figure S29). The lower impedance (22 kΩ) and higher exchange current density (9.54 μA cm−2) than those of symmetric cells inbaseline electrolyte (537 kΩ, 0.06 μA cm−2, respectively) can be also achieved (Figure 2k, Figure S30). Because of the functions in both electrolyte solvation structure and SEI, the PDA additive boosts the reversibility and kinetics of Mg anodes and consequently the whole Mg−CO2 battery cycling.
Conclusion
In conclusion, amine mediated chemistry strategy has been developed for improving the reversibility and kinetics of both cathode and anode in Mg−CO2 batteries. Through designing PDA assisted electrolyte, the reactants including CO2 and Mg2+ can be modulated through chemisorption and solvation shell regulation. Therefore, highly reversible and rapid redox conversion of CO2 and Mg have been realized on thermodynamic-kinetic levels. The PDA-mediated Mg−CO2 battery delivers excellent cyclic stability for 70 cycles (more than 400 h) with a discharge capacity of 600 mAh g−1 under a current density of 200 mA g−1 and high-rate performance varied from 100 to 2000 mA g−1 with 1.5 V overpotential. Even under low temperature of 0 °C or −15 °C, the system with PDA mediation can still operate normally. This demonstrated strategy provides a feasible scenario to realize a highly rechargeable and high-rate Mg−CO2 battery, which not only offers effective greenhouse gas utilization, but also provides the promising developments in multivalent metal-CO2 batteries.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No.22278308, 21875141, and 22179085); Natural Science Foundation of Tianjin (No. 22JCQNJC00210), and Beijing National Laboratory for Condensed Matter Physics. Open Access publishing facilitated by The University of Adelaide, as part of the Wiley - The University of Adelaide agreement via the Council of Australian University Librarians.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.