Ligand-Promoted Iron-Catalyzed Nitrene Transfer for the Synthesis of Hydrazines and Triazanes through N-Amidation of Arylamines
Shi-Yang Zhu
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorWen-Ji He
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorGuan-Chi Shen
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorDr. Zi-Qian Bai
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorFang-Fang Song
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorProf. Dr. Gang He
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hao Wang
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Gong Chen
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Frontiers Science Center for New Organic Matter, Nankai University, Tianjin, 300071 China
Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, 300192 China
Search for more papers by this authorShi-Yang Zhu
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorWen-Ji He
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorGuan-Chi Shen
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorDr. Zi-Qian Bai
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorFang-Fang Song
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorProf. Dr. Gang He
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hao Wang
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Gong Chen
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Frontiers Science Center for New Organic Matter, Nankai University, Tianjin, 300071 China
Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, 300192 China
Search for more papers by this authorGraphical Abstract
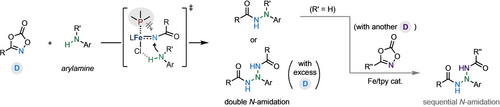
Bulky alkylphosphines (PtBu3) can switch the roles from actor to spectator ligands to promote the FeCl2-catalyzed N-amidation reaction of arylamines with dioxazolones leading to hydrazides in high efficiency and chemoselectivity. The new ligand-promoted N-amidation protocols offer a convenient way to access various challenging triazane compounds via double or sequential N-amidation of primary arylamines.
Abstract
Herein, we report that bulky alkylphosphines such as PtBu3 can switch the roles from actor to spectator ligands to promote the FeCl2-catalyzed N-amidation reaction of arylamines with dioxazolones, giving hydrazides in high efficiency and chemoselectivity. Mechanistic studies indicated that the phosphine ligands could facilitate the decarboxylation of dioxazolones on the Fe center, and the hydrogen bonding interactions between the arylamines and the ligands on Fe nitrenoid intermediates might play a role in modulating the delicate interplay between the phosphine ligand, arylamine, and acyl nitrene N, favoring N−N coupling over N−P coupling. The new ligand-promoted N-amidation protocols offer a convenient way to access various challenging triazane compounds via double or sequential N-amidation of primary arylamines.
Conflict of interest
The authors declare no competing financial interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202312465-sup-0001-misc_information.pdf7.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Synthesis of N-containing compounds:
- 1aJ. Emsley, Nature's Building Blocks: An A-Z Guide to the Elements, Oxford University Press, New York, 2nd Ed, 2011;
- 1bR. Hili, A. K. Yudin, Nat. Chem. Biol. 2006, 2, 284–287;
- 1cL. I. Belen′kii, Alkylnitrogen Compounds: Compounds with N−N, N−P, N−As, N−Sb, N−Bi, N−Si, N−Ge, N−B, and N-Metal Functional Groups, 2005, https://doi.org/10.1016/B0-08-044655-8/00029-5.
10.1016/B0-08-044655-8/00029-5 Google Scholar
- 2Selected reviews on metal-catalyzed nitrene transfer reactions:
- 2aH. M. Davies, J. R. Manning, Nature 2008, 451, 417–424;
- 2bJ. L. Roizen, M. Harvey, J. D. Bois, Acc. Chem. Res. 2012, 45, 911–922;
- 2cG. Dequirez, V. Pons, P. Dauban, Angew. Chem. Int. Ed. 2012, 51, 7384–7395;
- 2dJ. Egger, E. M. Carreira, Nat. Prod. Rep. 2014, 31, 449–455;
- 2eB. Du, C. M. Chan, C. M. Au, W. Y. Yu, Acc. Chem. Res. 2022, 55, 2123–2137.
- 3Selected examples of nitrene-mediated N-heteroatom bond formations:
- 3aO. G. Mancheño, C. Bolm, Org. Lett. 2006, 8, 2349–2352;
- 3bV. Bizet, L. Buglioni, C. Bolm, Angew. Chem. Int. Ed. 2014, 53, 5639–5642;
- 3cJ. Li, J. S. Cisar, D. Romo, Nat. Chem. 2013, 5, 510–517;
- 3dL. Maestre, R. Dorel, O. s Pablo, I. Escofet, W. M. Sameera, E. Álvarez, F. Maseras, M. M. Díaz-Requejo, A. M. Echavarren, P. J. Pérez, J. Am. Chem. Soc. 2017, 139, 2216–2223.
- 4Selected reviews on Fe catalysis:
- 4aC. Bolm, J. Legros, J. L. Paih, L. Zani, Chem. Rev. 2004, 104, 6217–6254;
- 4bI. Bauer, H. J. Knölker, Chem. Rev. 2015, 115, 3170–3387;
- 4cA. Fürstner, ACS Cent. Sci. 2016, 2, 778–789.
- 5Selected reviews of Fe-catalyzed nitrene-transfer reactions:
- 5aP. F. Kuijpers, J. I. van der Vlugt, S. Schneider, B. de Bruin, Chem. Eur. J. 2017, 23, 13819–13829;
- 5bP. Wang, L. Deng, Chin. J. Chem. 2018, 36, 1222–1240.
- 6Selected examples of Fe-catalyzed nitrene-mediated N-heteroatom bond formation. N−N bond formation:
- 6aB. J. Stokes, C. V. Vogel, L. K. Urnezis, M. Pan, T. G. Driver, Org. Lett. 2010, 12, 2884–2887;
- 6bY. Zhang, D. Duan, Y. Zhong, X. A. Guo, J. Guo, J. Gou, Z. Gao, B. Yu, Org. Lett. 2019, 21, 4960–4965; N−S bond formation:
- 6cH. Yu, Z. Li, C. Bolm, Angew. Chem. Int. Ed. 2018, 57, 12053–12056;
- 6dC. Lai, G. Mathieu, L. P. Gabrielli Tabarez, H. Lebel, Chem. Eur. J. 2019, 25, 9423–9426;
- 6eS. Chatterjee, S. Makai, B. Morandi, Angew. Chem. Int. Ed. 2021, 60, 758–765.
- 7Other selected Fe-catalyzed nitrene-mediated transformations:
- 7aR. Breslow, S. H. Gellman, J. Am. Chem. Soc. 1983, 105, 6728–6729;
- 7bG. S. Liu, Y. Q. Zhang, Y. A. Yuan, H. Xu, J. Am. Chem. Soc. 2013, 135, 3343–3346;
- 7cD. F. Lu, C. L. Zhu, Z. X. Jia, H. Xu, J. Am. Chem. Soc. 2014, 136, 13186–13189;
- 7dL. Legnani, B. Morandi, Angew. Chem. Int. Ed. 2016, 55, 2248–2251;
- 7eR. R. Anugu, S. Munnuri, J. R. Falck, J. Am. Chem. Soc. 2020, 142, 5266–5271.
- 8Selected reviews on N-based ligands in Fe catalysis:
- 8aC. Wei, Y. He, X. Shi, Z. Song, L. H. Pignolet, Coord. Chem. Rev. 2019, 385, 1–19;
- 8bA. Winter, U. S. Schubert, ChemCatChem 2020, 12, 2890–2941;
- 8cC. Damiano, P. Sonzini, E. Gallo, Chem. Soc. Rev. 2020, 49, 4867–4905.
- 9Selected reviews on phosphine ligand in catalysis:
- 9aL. H. Pignolet, Homogeneous Catalysis with Metal Phosphine Complexes, Plenum Press, New York and London, 1983, pp. 1–484;
- 9bJ. F. Hartwig, Organotransition Metal Chemistry: From Bonding to Catalysis, Univ Science Books, 2009;
- 9cR. Martin, S. L. Buchwald, Acc. Chem. Res. 2008, 41, 1461–1473.
- 10Selected examples of iron-phosphine complexes and phosphine ligand in Fe catalysis:
- 10aR. B. Bedford, M. Betham, D. W. Bruce, A. A. Danopoulos, R. M. Frost, M. Hird, J. Org. Chem. 2006, 71, 1104–1110;
- 10bN. S. Shaikh, K. Junge, M. Beller, Org. Lett. 2007, 9, 5429–5432;
- 10cR. Shang, L. Ilies, E. Nakamura, J. Am. Chem. Soc. 2015, 137, 7660–7663;
- 10dS. Liang, X. Zhao, T. Yang, W. Yu, Org. Lett. 2020, 22, 1961–1965;
- 10eL. Adak, T. Hatakeyama, M. Nakamura, Bull. Agric. Chem. Soc. Jpn. 2021, 94, 1125–1141;
- 10fK. B. Renkema, M. Ogasawara, W. E. Streib, J. C. Huffman, K. G. Caulton, Inorg. Chim. Acta 1999, 317, 226–230.
- 11Our previous Fe- and Ir-catalyzed nitrene-mediated N−N coupling reactions:
- 11aH. Wang, H. Jung, F. Song, S. Zhu, Z. Bai, D. Chen, G. He, S. Chang, G. Chen, Nat. Chem. 2021, 13, 378–385;
- 11bF. Song, S. Zhu, H. Wang, G. Chen, Chin. J. Org. Chem. 2021, 41, 4050–4058.
- 12Other related metal-catalyzed nitrene-mediated N−N coupling reactions:
- 12aM. Kono, S. Harada, T. Nemoto, Chem. Eur. J. 2019, 25, 3119–3124;
- 12bJ. P. Barbor, V. N. Nair, K. R. Sharp, T. D. Lohrey, S. E. Dibrell, T. K. Shah, M. J. Walsh, S. E. Reisman, B. M. Stoltz, J. Am. Chem. Soc. 2023, 145, 15071–15077.
- 13Selected reviews on 1,4,2-dioxazol-5-ones:
- 13aY. Park, Y. Kim, S. Chang, Chem. Rev. 2017, 117, 9247–9301;
- 13bT. Shimbayashi, K. Sasakura, A. Eguchi, K. Okamoto, K. Ohe, Chem. Eur. J. 2019, 25, 3156–3180;
- 13cK. M. van Vliet, B. de Bruin, ACS Catal. 2020, 10, 4751–4769;
- 13dS. Y. Hong, Y. Hwang, M. Lee, S. Chang, Acc. Chem. Res. 2021, 54, 2683–2700.
- 14Selected examples of dioxazolone-mediated reactions:
- 14aY. Park, K. T. Park, J. G. Kim, S. Chang, J. Am. Chem. Soc. 2015, 137, 4534–4542;
- 14bS. Y. Hong, Y. Park, Y. Hwang, Y. B. Kim, M.-H. Baik, S. Chang, Science 2018, 359, 1016–1021;
- 14cH. Wang, G. Tang, X. Li, Angew. Chem. Int. Ed. 2015, 54, 13049–13052;
- 14dY. J. Liang, Y. F. Liang, C. H. Tang, Y. Z. Yuan, N. Jiao, Chem. Eur. J. 2015, 21, 16395–16399;
- 14eR. Mei, J. Loup, L. Ackermann, ACS Catal. 2016, 6, 793–797;
- 14fX. Wang, A. Lerchen, F. Glorius, Org. Lett. 2016, 18, 2090–2093;
- 14gK. M. van Vliet, L. H. Polak, M. A. Siegler, J. I. van der Vlugt, C. F. Guerra, B. de Bruin, J. Am. Chem. Soc. 2019, 141, 15240–15249;
- 14hH. Lei, T. Rovis, J. Am. Chem. Soc. 2019, 141, 2268–2273;
- 14iH. Wang, Y. Park, Z. Bai, S. Chang, G. He, G. Chen, J. Am. Chem. Soc. 2019, 141, 7194–7201;
- 14jZ. Bai, S. Zhu, Y. Hu, P. Yang, X. Chu, G. He, H. Wang, G. Chen, Nat. Commun. 2022, 13, 6445.
- 15Fe-catalyzed nitrene-mediated N−P coupling reactions:
- 15aZ. Bai, F. Song, H. Wang, W. Cheng, S. Zhu, Y. Huang, G. He, G. Chen, CCS Chem. 2022, 4, 2258–2266; For a related report:
- 15bJ. J. Tang, X. Yu, Y. Wang, Y. Yamamoto, M. Bao, Angew. Chem. Int. Ed. 2021, 60, 16426–16435.
- 16Selected reviews on polynitrogen synthesis and recent examples:
- 16aL. M. Blair, J. Sperry, J. Nat. Prod. 2013, 76, 794–812;
- 16bA. J. Waldman, T. L. Ng, P. Wang, E. P. Balskus, Chem. Rev. 2017, 117, 5784–5863;
- 16cJ. F. Liebman, A. Greer, The Chemistry of Nitrogen-Rich Functional Groups, New York, USA. John Wiley & Sons Ltd, 2020.
- 17Selected reports on azanes including triazanes:
- 17aV. N. Egger, L. Hoesch, A. S. Dreiding, Helv. Chim. Acta 1983, 66, 1599–1607;
- 17bR. M. Richard, D. W. Ball, J. Mol. Model. 2008, 14, 29–37;
- 17cM. Forstel, P. Maksyutenko, B. M. Jones, B. J. Sun, S. H. Chen, A. H. Chang, R. I. Kaiser, ChemPhysChem 2015, 16, 3139–3142;
- 17dA. M. Prokhorov, P. E. Prokhorova, Prog. Heterocycl. Chem. 2015, 27, 451–464;
- 17eE. Lacôte, L. Joucla, A. Glowacki, V. Jeux, G. Gasnier, G. Jacob, Synlett 2018, 29, 566–570;
- 17fI. Avigdori, A. Pogoreltsev, A. Kaushanski, N. Fridman, M. Gandelman, Angew. Chem. Int. Ed. 2020, 59, 23476–23479;
- 17gW. Liu, Q. Jiang, X. Yang, Angew. Chem. Int. Ed. 2020, 59, 23598–23602.
- 18For selected reviews on constructing N−N bonds:
- 18aU. Ragnarsson, Chem. Soc. Rev. 2001, 30, 205–213;
- 18bE. Licandro, D. Perdicchia, Eur. J. Org. Chem. 2004, 665–675;
- 18cQ. Guo, Z. Lu, Synthesis 2017, 49, 3835–3847;
- 18dA. Tabey, P. Y. Vemuri, F. W. Patureau, Chem. Sci. 2021, 12, 14343–14352.
- 19Other selected examples of intermolecular N−N coupling reactions:
- 19aB. R. Rosen, E. W. Werner, P. S. Baran, J. Am. Chem. Soc. 2014, 136, 5571–5574;
- 19bM. C. Ryan, J. R. Martinelli, S. S. Stahl, J. Am. Chem. Soc. 2018, 140, 9074–9077;
- 19cG. Li, S. P. Miller, A. T. Radosevich, J. Am. Chem. Soc. 2021, 143, 14464–14469.
- 20Condensation or rearrangement reaction of dioxazolones:
- 20aP. Dubé, N. F. F. Nathel, M. Vetelino, M. Couturier, C. L. Aboussafy, S. Pichette, M. L. Jorgensen, M. Hardink, Org. Lett. 2009, 11, 5622–5625;
- 20bD. E. Polat, D. D. Brzezinski, A. M. Beauchemin, Org. Lett. 2019, 21, 4849–4852.
- 21
- 21aC. Roullier, M. Chollet-Krugler, P. Weghe, F. L. Devehat, J. Boustie, Bioorg. Med. Chem. Lett. 2010, 20, 4582–4586;
- 21bG. Le Goff, J. Ouazzani, Bioorg. Med. Chem. 2014, 22, 6529–6544.
- 22The mechanism of the N-amidation of hydrazides with dioxazolones under tpy-promoted conditions has not been examined. At the moment, it is unclear why the separate reaction of hydrazide with dioxazolones gives a lower yield of triazane than the one-pot double N-amidation setting. As shown in Figure 3B, intermediate 5FeP-pdt2 needs to undergo protonolysis to release the final hydrazide product 3 a. We speculate that 3 a might be able to react with another dioxazolone to give the triazane product. Formation of intermediate 5FeP-pdt2 from hydrazide might be difficult under our reaction conditions. Nevertheless, the triazane formation pathway via a nucleophilic attack of hydrazide to phosphine- or L6-bound Fe acyl nitrene intermediates cannot be ruled out.
- 23For two closely related N−H⋅⋅⋅Cl hydrogen bonding in Fe complexes:
- 23aG. A. Tondreau, D. A. Sweigart, Inorg. Chem. 1984, 23, 1060–1065;
- 23bD. Sahoo, M. G. Quesne, S. P. de Visser, S. P. Rath, Angew. Chem. Int. Ed. 2015, 54, 4796–4800; For hydrogen bonding with chloride anion in other classes of metal complexes:
- 23cB. Ahrens, P. G. Jones, A. K. Fischer, Eur. J. Inorg. Chem. 1999, 1103–1110;
10.1002/(SICI)1099-0682(199907)1999:7<1103::AID-EJIC1103>3.0.CO;2-0 CAS Web of Science® Google Scholar
- 23dJ. R. Wilson, M. Zeller, N. K. Szymczak, Chem. Commun. 2021, 57, 753–756.
- 24
- 24aE. R. King, E. T. Hennessy, T. A. Betley, J. Am. Chem. Soc. 2011, 133, 4917–4923;
- 24bE. T. Hennessy, R. Y. Liu, D. A. Iovan, R. A. Duncan, T. A. Betley, Chem. Sci. 2014, 5, 1526–1532;
- 24cJ. Kweon, S. Chang, Angew. Chem. Int. Ed. 2021, 60, 2909–2914;
- 24dL. Zhang, L. Deng, Chin. Sci. Bull. 2012, 57, 2352–2360.
- 25We did not locate any suitable transition states for an N−N coupling process that does not involve any hydrogen-bonding interaction between PhNH2 and 5Fe(=N)P.
- 26Deposition numbers 2166614 (for 62) and 2254336 (for 63) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 27Our preliminary energy decomposition analyses (EDA) showed that 5FeP(=N)N′-TS2 has a stronger total interaction energy than 5FeP(=N)N′-TS1 (−20.5 vs −10.9 kcal/mol, see more details in SI).