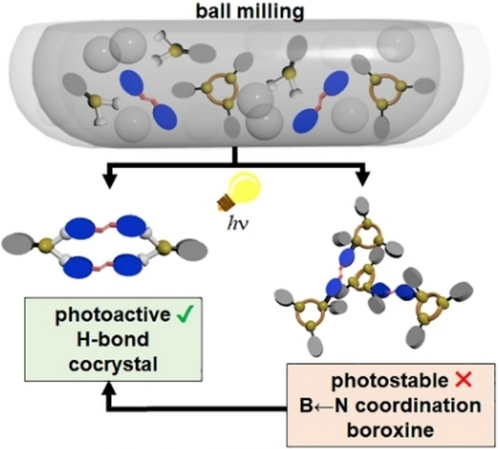
Mechanochemical Mediated Coexistence of B←N Coordination and Hydrogen Bonding
Graphical Abstract
Abstract
Mechanochemistry afforded a photoactive cocrystal via coexisting (B)O−H⋅⋅⋅N hydrogen bonds and B←N coordination. Specifically, solvent-free mechanochemical ball mill grinding and liquid-assisted grinding of a boronic acid and an alkene resulted in mixtures of hydrogen-bonded and coordinated complexes akin to mixtures of noncovalent complexes that can be obtained in solution in equilibria processes. The alkenes of the hydrogen-bonded assembly undergo an intermolecular [2+2] photodimerization in quantitative conversion, effectively reporting the outcome of the self-assembly processes. Our results suggest that interplay involving noncovalent bonds subjected to mechanochemical conditions can lead to functional solids where, in the current case, the structure composed of the weaker hydrogen bonding interactions predominates.
Mechanochemistry provides exciting synthetic opportunities to generate novel functional solid phases and mixtures. The approach allows for the construction of supramolecular architectures predicated to form by directional noncovalent forces such as hydrogen bonding (e.g., cocrystals) and coordination (e.g., B-nitrogen).1-5 The use of mechanochemical force to drive chemical reactivity can promise not only more green and efficient processes to be developed for chemical syntheses but also access to alternative reactivity and mechanistic materials science pathways (e.g., catalytic).3, 6-8 A similar argument has been hypothesized for new mechanochemical pathways in prebiotic chemistry from inactivated amino acids in extraterrestrial and terrestrial collisions.9-11
Boronic acids (BAs) and related derivatives (e.g., boronic esters, boroxines) are unique and intriguing platforms from which to study self-assembly. As a building block in a supramolecular synthesis, the B(OH)2 group of an aryl BA can react to participate in hydrogen bonds12-18 (i.e., O−H⋅⋅⋅X) and/or B-coordination (i.e., B−X),5, 18-23 depending on reaction conditions. Thus, hydrogen-bonded cocrystals of boronic acids will typically form from solution at room temperature14-17 while boroxines typically form with addition of heat23-25 or added reaction time.22 Moreover, the dual nature of BAs in participating in different types of noncovalent bonds can effectively enable BAs to provide information on chemical environment in a dynamic self-assembly process.
Herein we demonstrate that when subjected to continuous mechanochemical stimulus at room temperature, molecular building blocks (ba=phenylboronic acid; bpe=trans-1,2-bi-(4-pyridyl)ethylene) of multicomponent crystals undergo self-assembly to involve coexistence of the hydrogen-bonded and coordination complexes [(ba)(bpe)] (1) and [(Ph3B3O3)2(bpe)] (2), respectively. Continuous grinding results in the exclusive generation of the hydrogen-bonded structure (Scheme 1) with the alkene components assembled and preorganized for an intermolecular [2+2] photodimerization in the crystalline state. Upon exposure to UV-radiation, 1 affords 4,4′-tpcb stereospecifically and in quantitative yield. Density functional theory (DFT) calculations demonstrate a high-packing density as a driving force to form the hydrogen-bonded complex versus B←N coordination as the final product. It is, thus, the generation of the more weakly-bound hydrogen-bonded complex 1 that evolves during the mechanochemically mediated conversion of the self-assembled structures as the final crystalline product. Despite a broad body of B-based self-assembled structures reported in the literature, we are unaware of an example wherein B←N coordination and B(OH)2 hydrogen bonding have been simultaneously generated and undergo conversion under mechanochemical conditions.
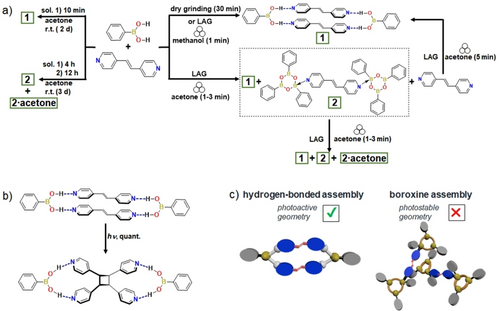
Mechanochemistry leading to coexisting complexes: (a) assemblies prepared involving ba and bpe (1 : 1 ratio) using ball mill dry grinding and LAG conditions, (b) [2+2] photodimerization of 1, and (c) photoactive and photostable geometries of 1 and 2.
Both 1 and 2 were formed according to literature precedents. Pedireddi has described the self-assembly of ba and bpe to generate the cocrystal hydrate [(ba)(bpe)⋅H2O]12 by dissolution at room temperature. The components form a ladder-like hydrogen-bonded structure. Zhang has described the synthesis of the boroxine [(Ph3B3O3)2(bpe)]⋅nC6H623 by dissolution with heating. The boroxine forms a host–guest solid with benzene.
When ba and bpe (1 : 1 ratio) were together dissolved in acetone at room temperature, colorless crystals of 1 as irregular prisms formed after allowing the solution to stir at room temperature (10 minutes) and then slowly evaporate (2 days).
A single-crystal X-ray diffraction (SCXRD) analysis of 1 revealed the components to crystallize in the monoclinic space group P21/c, with the asymmetric unit composed of full ba and bpe molecules (Figure S1, Supporting Information).26 The structure is composed of tetra-molecular assemblies sustained by (B)O−H⋅⋅⋅Npyr hydrogen bonds [O⋅⋅⋅N, 2.802(2) Å/2.764(2) Å; ∠O−H⋅⋅⋅N, 162°/150°], which contrasts the reported hydrate [(ba)(bpe)⋅H2O] based on catemers.12 The acid groups of 1 adopt a syn-syn conformation (Figure 1a), with the pyridyl groups and phenyl groups of ba approximately orthogonal (75°). The B(OH)2 groups are nearly coplanar (4.36°) with the phenyl rings. As a result of the assembly process, the bpe stacks with the C=C bonds preorganized for a [2+2] photodimerization (centroid-centroid distance 3.78(2) Å; N⋅⋅⋅N 3.34(2) Å). Edge-to-face packing is also present and sustained by C−H⋅⋅⋅π (C⋅⋅⋅π 3.68–3.71 Å) interactions (Figure 1b).
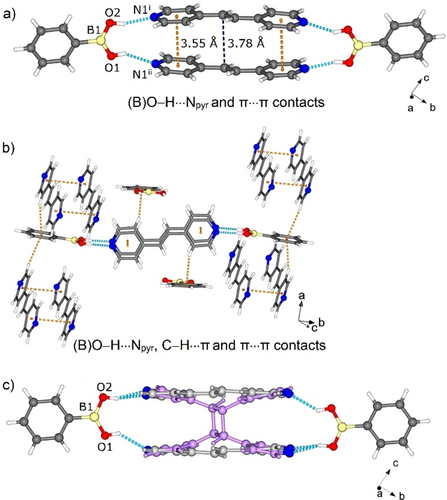
X-ray structure 1: (a) assemblies with O−H⋅⋅⋅N hydrogen bonds, (b) edge-to-face packing, and (c) formation of 4,4′-tpcb in 1. Symmetry operators: (i) 1-x, 0.5+y, 1.5-z; (ii) x, 2.5-y, 0.5+z.
When a crystalline powder of 1 was subjected to UV irradiation (48 h), bpe formed 4,4′-tpcb in near quantitative yield (97 % yield) (Figure S4, NMR data Supporting Information). Optical microscopy suggested the crystals to react in a single-crystal-to-single-crystal (SCSC) transformation. When single crystals of 1 were subjected to UV irradiation for 3 h (450 W, medium pressure Hg-lamp), the photoreaction proceeded as a SCSC reaction (4,4′-tpcb yield 53 % in crystal) (Figure 1c). In the solid, 4,4′-tpcb interacts with ba via (B)O−H⋅⋅⋅Npyr hydrogen bonds [O⋅⋅⋅N, 2.82(2) Å/2.720(1) Å; ∠O−H⋅⋅⋅N, 166°/141°] with the acid group in the syn-syn conformation.
The boroxine 2 was obtained according to Höpfl.22 The acid ba was initially dissolved in acetone and stirred at room temperature (4 hours). The alkene bpe was then added to the solution (1 : 1 ratio), which was allowed to stir (12 hours). Upon slow evaporation (3 days), a mixture of colorless blade- 2 and plate-shaped crystals 2⋅acetone formed.
A SCXRD analysis of the blade crystals 2 revealed the components to crystallize in the monoclinic space group P21/n.23 The asymmetric unit consists of the boroxine ring system and a half bpe molecule (Figure S2, X-ray data Supporting Information). The assembly process generated a 2 : 1 adduct (B←N bond 1.643(4) Å, THC 78.8 %) with the terminal phenyl groups in an anti-conformation (Figure 2a).27 Neighboring adducts assemble herringbone via C−H⋅⋅⋅π (C⋅⋅⋅π 3.34–3.74 Å) and π⋅⋅⋅π (3.62 Å) interactions (Figure 2b). In contrast to 1, the solid 2 is photostable, with the alkene packing with nearest-neighbor C=C bonds separated by 10.94 Å (Figure S6, NMR data Supporting Information).
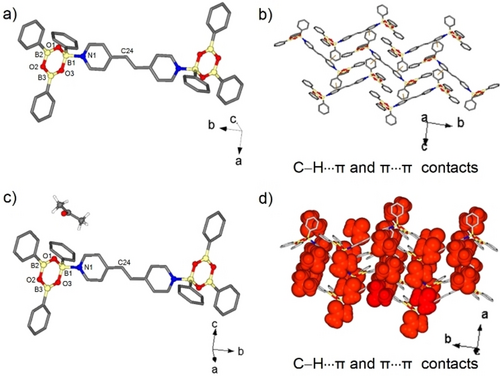
X-ray structure of 2 and 2⋅acetone: (a, b) B←N adduct of 2 and 2D strands sustained by C−H⋅⋅⋅π and π⋅⋅⋅π contacts; (c,d) B←N adduct of 2⋅acetone showing 2D arrangement sustained by C−H⋅⋅⋅π and π⋅⋅⋅π contacts and inclusion of acetone. For clarity, hydrogen atoms were omitted.
Whereas the hydrogen-bonded assembly and boroxine involving ba and bpe require different conditions to form from solution, both 1 and 2 form and coexist when the starting materials (1 : 1 ratio) are subjected to liquid assisted (LAG) grinding. Thus, when ba and bpe were subjected to LAG with acetone (50 μL), 1 and 2 rapidly formed within 1 to 3 min as revealed by PXRD. Crystalline, or ‘free’, bpe was also present in the solid. Hydrogen-bonded 1, however, formed directly using LAG with methanol or dry grinding. Specifically, 1 formed quantitatively within 1 min using LAG with methanol and 30 min with dry grinding (Figure 3a). Continuous LAG with acetone did result in the predominant formation of 1 in 5 min with only traces of 2 present (Figure 3b). We note that grinding ba and bpe in a 6 : 1 ratio by LAG using acetone generated 2 as the sole product in 30 min (Figure S13, X-ray data Supporting Information). Other common liquids (e.g., acetonitrile, water) resulted in partial generation of 1 and only starting materials using LAG in periods of up to 30 min (see ESI).
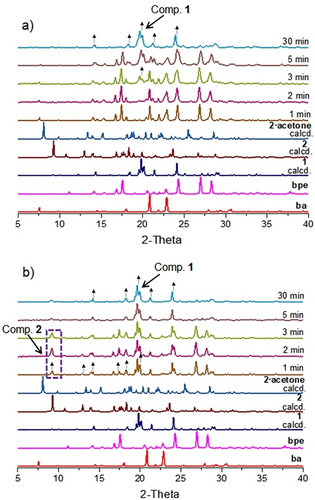
PXRD patterns of ba and bpe products (1 : 1 ratio): (a) dry grinding (b) LAG with acetone (50 μL).
The ground solid composed of both 1 and 2 that forms by LAG with acetone is photoactive. UV irradiation of the solid ground for 2 mins generated 4,4′-tpcb in up to 71 % yield (48 h). The PXRD data prior to the photoreaction was consistent with 1 and 2 present in comparable amounts. Grinding the photoreacted solid and further irradiation (60 h) generated 4,4′-tpcb in 90 % yield. Prolonged irradiation (12 h) resulted in 4,4′-tpcb generated in 95 % yield (Figure S5, Supporting Information).
When a sample containing 1 and 2 prepared independently and then mixed (1 : 1 ratio) was subjected to LAG with acetone (50 μL) (1 to 3 min) the relative amounts 1 and 2 did not significantly change. Diffraction peaks (e.g., 2θ=8.0, 20.9) ascribed to a new phase, however, did appear (see also, Supporting Information). Dry grinding of the preformed mixture for up to 30 min also revealed 1 and 2 to remain as predominant phases. Conversion of the solid to only the hydrogen-bonded complex 1, thus, did not occur as with the case involving grinding of the starting materials. However, continuous LAG of the preformed mixture of 1 and 2 prepared as 1 : 1 and 0.70 : 0.30 using water and acetone/water 1 : 1 (v/v), respectively, afforded 1 as the final product. The observation is consistent with a role of water to influence hydrolysis of the boroxine of 2 to form the cocrystal 1 (Figures S16–S17, Supporting Information).
We identified the new phase formed upon LAG of preformed 1 and 2 as the acetone solvate 2⋅acetone. A SCXRD analysis of the plate-like crystals that formed in the synthesis of 2 revealed the structure as 2⋅acetone (Figure 2a). The boroxine and acetone crystallize in the monoclinic space group P21/n, with the components stabilized by C−H⋅⋅⋅π (C⋅⋅⋅π 3.64–3.82 Å) and π⋅⋅⋅π (3.69 Å) interactions that give layers that host the acetone guests (Figure 2b–d). The solids 2 and 2⋅acetone are isoskeletal with 2 effectively being the apohost.
That hydrogen-bonded 1 is the final product of the LAG experiments can be rationalized on packing considerations. Analyses of Hirschfeld 2D fingerprint plots reveal the contributions of C⋅⋅⋅H for 1 (ca. 32 %) to be greater than 2 (ca. 29 %) and 2⋅acetone (ca. 27 %) (Figures S21–S23, Supporting Information).28 The contributions comprise C−H⋅⋅⋅π interactions.29 The prevalence of the interactions is corroborated with energy framework calculations (B3LYP/6-31G(d,p) level) using Crystal Explorer.28, 30, 31 Electrostatic energy of 1 dominates versus polarization (Table 1 and Figure S24, Supporting Information). The packings of 2 and 2⋅acetone involve more dispersion energy, which are ascribed to increased π-stacking and less hydrogen bonding and C−H⋅⋅⋅π interactions. The contributions of N⋅⋅⋅H (ca. 12 %) and O⋅⋅⋅H (ca. 7 %) for 1 are, as expected, significantly greater than 2 and 2⋅acetone. We believe these observations are important since the calculations suggest crystal packing to be a determining factor that dictates the high selectivity in the outcome of a self-assembly process (i.e., hydrogen bonding versus B←N coordination) mediated by mechanochemistry. The cohesive energy of crystal packing presumably dominates in the self-assembly process to allow for hydrogen-bonded 1 to form exclusively as the final product versus B←N coordinated 2.
Compound |
Eele |
Epol |
Edis |
Erep |
Etotal |
|---|---|---|---|---|---|
1 |
−146 |
−40.3 |
−245.1 |
262 |
−235.7 |
2 |
−53.8 |
−28.1 |
−282.9 |
151.9 |
−230.2 |
2⋅acetone |
−68.3 |
−36.8 |
−329.2 |
178 |
−276.3 |
In this report, we have demonstrated hydrogen bonds and B←N coordination to coexist in a self-assembly mediated by mechanochemistry using dry grinding and liquid-assisted conditions. Continuous grinding afforded a photoactive hydrogen-bonded assembly as the sole self-assembled product. Dynamic interplay between noncovalent bonds under mechanochemical conditions, akin to solution equilibria, can likely be expected in similar B-based materials and, more generally, self-assembling structures.32, 33 We are currently focused on exploiting the formation of mixed solids generated by competitive milling to control the structures and functions supramolecules.33 Further boundaries of supramolecular and coordination chemistries of boron remain to be explored for the design of functional and smart materials (e.g., self-healing, mechanical) and to provide an extended picture of supramolecular and mechanochemical systems.
Acknowledgments
The authors thank the National Science Foundation (NSF DMR-1708673, DMR-2221086, and CHE-1828117) for financial support. M. G. V.-R. is grateful to Secretaría de Educación, Ciencia, Tecnología e Innovación (SECTEI) de la Ciudad de México (CDMX) and Consejo Nacional de Ciencia y Tecnología (CONACyT) for Postdoctoral Fellowships. G.C.-A. is grateful to the M. J. Murdock Charitable Trust (NS-202222588, and FSU-202118942), and Reed College (start-up and Stillman Drake funds) for financial support.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in Cambirdge Structural Database at https://www.ccdc.cam.ac.uk/, reference number 2255582.