Evidence of a Uranium-Paddlewheel Node in a Catecholate-Based Metal–Organic Framework
Graphical Abstract
Through solvothermal combination of uranyl and a redox-active linker, we reduced the U−O bonds and subsequently formed a metal-organic framework built from a uranium paddlewheel node. This paddlewheel node, where two uranium atoms are connected by four hexahydroxytriphenylene species and capped with water moieties, is unprecedented.
Abstract
The interactions between uranium and non-innocent organic species are an essential component of fundamental uranium redox chemistry. However, they have seldom been explored in the context of multidimensional, porous materials. Uranium-based metal–organic frameworks (MOFs) offer a new angle to study these interactions, as these self-assembled species stabilize uranium species through immobilization by organic linkers within a crystalline framework, while potentially providing a method for adjusting metal oxidation state through coordination of non-innocent linkers. We report the synthesis of the MOF NU-1700, assembled from U4+-paddlewheel nodes and catecholate-based linkers. We propose this highly unusual structure, which contains two U4+ ions in a paddlewheel built from four linkers—a first among uranium materials—as a result of extensive characterization via powder X-ray diffraction (PXRD), sorption, transmission electron microscopy (TEM), and thermogravimetric analysis (TGA), in addition to density functional theory (DFT) calculations.
Introduction
Uranium is an element rich in oxidation states; reports exist of coordination complexes of uranium in every oxidation state between +1 and +6.1-8 Each oxidation state is of fundamental interest due to the associated unique behavior in several regimes. For instance, U3+ complexes may reduce the highly stable nitrogen-nitrogen triple bond within molecular N2 at ambient conditions,3 in addition to other challenging reactions,9 while U5+ and U6+ species are important players in photocatalytic transformations.10 However, studying complexes of uranium to explore fundamental reactivity, especially those that do not contain the stable uranyl ion ([UO2]2+), is not trivial. In addition to lacking air and moisture stability,11 U1+, U2+, and U3+ generally require sterically bulky, synthetically challenging ligands to prevent unwanted side reactions and to provide necessary electron donation during a chemical reaction,12, 13 limiting substrate access to the uranium centers. Additionally, the starting materials for low-valent uranium species are typically UX3 salts (X=Cl, I) and their derivatives, which are commonly derived from dissolving pyrophoric uranium turnings in strong acids.14 Furthermore, homogeneous U5+ is prone to disproportionation, wherein two U5+ species collide and experience spontaneous redox to form a U6+ moiety and a generally insoluble U4+ species. As controlling uranium's oxidation state is necessary for tuning its chemical properties, several groups have sought to adjust uranium's oxidation state via interactions with redox-active ligands.15-18
Approaching the stabilization of uranium in unusual oxidation states and studying its redox-activity through the addition of redox-active species is quite adaptable for use within metal-organic frameworks (MOF). As self-assembled, extended structures constructed from inorganic nodes and organic linkers, MOFs are crystalline, tunable, and porous.19 These properties translate well to uranium incorporation for fundamental study;20 the organic linkers, typically small, functionalizable molecules, can immobilize uranium-based nodes to prevent interaction and disproportionation. The crystallinity inherent to MOFs also makes them suitable for X-ray diffraction to determine structural characteristics of the framework. Additionally, the innate porosity and multidimensionality of MOFs can enable efficient diffusion of substrates through the framework, ensuring accessibility of substrates to the uranium center during catalytic reactions. Incorporation of uranium onto the nodes,21 linkers,22 and as the node itself23 of MOFs and coordination polymers (CPs) is already well-described within the literature, with the U4+ and U6+ oxidation states being most common.
Several examples of established uranium coordination motifs are shown in Figure 1. Tetravalent uranium primarily forms oxygen-containing clusters; for instance, hexanuclear U4+-oxo clusters and benzene dicarboxylate linkers form the uranium analogue of UiO-66.24 Additionally, while they have not yet been incorporated into a MOF, U4+-oxo clusters come in U13, U16, U24, and even U38 varieties.25 However, by far the most common uranium bonding motif in MOFs and CPs is uranyl, wherein a U6+ atom is bound axially by two oxygens in a highly covalent manner, leaving ionic bonding sites open in the equatorial plane.20 While the 8-coordinated, single uranyl motif is the most common in MOFs, reports do exist of square pyramidal and pentagonal pyramidal uranyl as part of MOF building blocks.17, 26 Regardless of whether uranium is present as a cluster or single uranyl ion, the synthetic pathways for achieving unusual uranium oxidation states in MOFs are not established. Additionally, the generally high coordination numbers of both U4+-oxo clusters and uranyl still inhibit substrate access to uranium, meaning that more work must be done to stabilize unusual uranium oxidation states in MOFs, either through de novo or post-synthetic means.
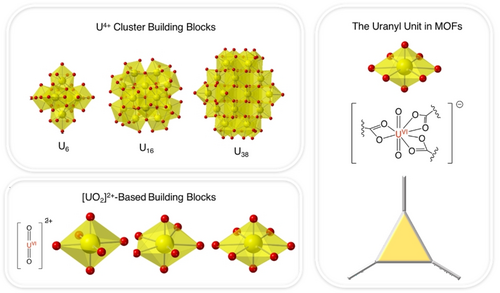
Left top: Previously reported tetravalent uranium-oxo clusters of different sizes. Left bottom: Previously reported uranyl coordination motifs. Right: The 8-coordinated uranyl unit and its corresponding planar topological building block. Color key for polyhedra: U (yellow), O (red).
Previous work has shown that incorporating catecholates, a comparatively understudied linker family, can endow MOFs with interesting electronic and redox activity that is not accessible to carboxylate-based MOFs.27, 28 The linker hexahydroxytriphenylene (HHTP), which is planar and has three catechol binding sites, has been a primary component in many redox-focused studies29-32 due to its seven possible oxidation states.33 With one and two-electron reductions a strong possibility for metals bound to HHTP, and given the propensity of uranyl ions to be reduced in mild conditions (e.g. visible light, with mild acids and heat) and with redox-active linkers,34, 35 as previously discussed, we originally hypothesized that combining uranyl and HHTP together would result in a system with both tunable uranium oxidation states, as well as novel coordination motifs due to the catecholate's larger bite angle than that of a carboxylic acid. In our efforts, the formation of an unexpected coordination motif in NU-1700 was discovered. NU-1700 is a proposed tbo-topology MOF assembled from uranium-paddlewheel nodes and HHTP linkers. Unfortunately, large crystals of the cubic, nanoscale particles of this MOF, in our hands, could not be obtained. Thus, we utilized a fleet of characterization techniques, including powder X-ray diffraction (PXRD) analysis, density functional theory (DFT) calculations, transmission electron microscopy (TEM), and thermogravimetric analysis (TGA) to propose the structure of this unusual material. To the best of our knowledge, this is one of few reports of a uranium paddlewheel, the first example of a non-uranyl MOF formed from uranyl, and one of only a handful of three-dimensional catecholate-based frameworks.
Results and Discussion
NU-1700 was synthesized solvothermally via mixture of uranyl nitrate hexahydrate, HHTP, and tetrabutylammonium bromide as a templating agent in N,N-dimethylformamide (DMF) with trifluoroacetic acid as a modulator. Scanning electron microscopy (SEM) images demonstrate cubic particles with particle sizes of 0.5–0.8 μm (Figures 2A and S1). In our hands, NU-1700 only showed this size range of particles, prohibiting the use of single-crystal X-ray diffraction for structural characterization. The PXRD pattern of the black powder demonstrated low angle peaks starting at 2θ=4.7°, corresponding to a d-spacing of approximately 18.6 Å (Figure 2B). This spacing, combined with N2 isotherms and pore-size distributions showing approximately 12 Å pores (Figure S2), demonstrate that NU-1700 is a crystalline, porous material. Unit cell analysis of the PXRD pattern indicates a cubic unit cell, consistent with the morphology of the particles; specifically, and the unit cell parameters a=b=c=36.904 Å (Table S1 and Figure S3). These diffraction peaks are quite similar to those seen in nano-sized HKUST-1, a tbo-topology Cu-paddlewheel framework containing a planar, tritopic linker in the space group Fm-3m.36 Of note are the first five diffraction peaks; as these peaks correspond to the largest d-spacings, their position generally indicates the void spaces caused by the connectivity of the building blocks within the framework. The similarity of these five peaks in both relative position and relative intensity strongly suggests that NU-1700 is also a tbo-topology framework. It is important to note that, regardless of topology, nano-sized MOFs exhibit different relative peak intensities as compared to micro-sized MOFs due to preferred orientation;37, 38 therefore, we considered nano-HKUST-1 rather than standard HKUST-1.
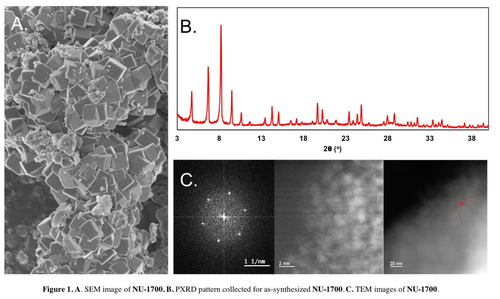
A) SEM image of NU-1700 crystallites, B) PXRD pattern of as-synthesized NU-1700, C) TEM images of NU-1700, demonstrating lattice elements, distances between large diffraction centers, and interplanar angles.
These findings cannot be consistent with a uranyl node. The uranyl node is generally characterized by three carboxylate linkers coordinated to U6+ in the equatorial plane, thus forming a planar building block (Figure 1) with only slight distortion into the axial plane possible. As the tbo topology requires a planar tritopic and a three-dimensional tetratopic building unit, with the tritopic unit corresponding to HHTP, whatever node is present in NU-1700 must meet the following criteria: 1) it must be 4-connected in nature and 2) it must have a geometry that induces three-dimensionality. The resulting uranyl node would thus require four catecholate linkers to sit heavily distorted within the equatorial plane. While Shuao et al. have recently reported the binding of three catecholate sites to uranyl in the equatorial plane,39 four would require significant distortion.
Instead, we hypothesized that a four-connected uranium-based node, not uranyl-based, was present in NU-1700. To confirm this, we utilized air-free diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) to verify the presence, or lack thereof, of HHTP-coordinated uranyl. We did not find conclusive evidence of the asymmetric uranyl-oxo stretching vibration (Figure S4), as the peaks present in the characteristic uranyl region (910 to 930 cm−1) are also present in the infrared spectrum of HHTP.40 Furthermore, we employed solid-state electron paramagnetic resonance (EPR) spectroscopy at 77 K to look for signs of redox in the node and linker (Figure S5) that would imply the conversion of U6+ to a lower oxidation state. We observed a strong organic radical signal at 340 mT, corresponding to the semiquinone form of the HHTP linker; the slight asymmetry may be attributed to bonding interactions between uranium and HHTP. We also observed a weak, rhombic signal, which we assigned as possibly half-field transition of two coupled HHTP radicals.
The presence of U4+ within the system was further corroborated by solid-state UV/Vis; three bands were centered at 450, 525, and 600 nm (Figures S6 and S7), which are the approximate wavelengths associated with the 5f16s1→5f2 electron transitions for U4+ in the visible region.41, 42 We attribute the blue-shifted and broadened wavelengths, relative to homogeneous U4+, to the solid-state, more complex electronic environment. Additionally, cyclic voltammetry (CV) experiments (Figure S8) demonstrated that while the anodic region is dominated by HHTP oxidations, with peaks at 1.4 and 1.7 V vs. Ag/AgCl corresponding to the reversible and irreversible oxidations to the semiquinone and diketone forms,43 peaks in the cathodic region at −1.85 and −2.3 V vs. Ag/AgCl potentially correspond to a U4+ reduction and a ligand-centered reduction, respectively.44 Furthermore, X-ray photoelectron spectroscopy (XPS) experiments of NU-1700 over the course of two days suggested potential oxidation of U atoms within the framework. Relative to the C=O, N−O, and C−O bonds, which may be assigned as residual nitrate groups from the UO2(NO3)2 starting salt remaining in the pores and as the C−O bonds of the linker, the U−O bonds became more prevalent over the course of 24 hours (Figure S9). These pieces of evidence taken together with the PXRD analysis point towards a U4+-containing node, implying solvothermal reduction of uranyl during synthesis.
Given the inability to characterize NU-1700 via single crystal X-ray diffraction, we deferred to alternate characterization methods to determine topology and node structure. For more concrete, experimental evidence of the tbo topology independent of PXRD analysis, we used TEM to identify lattice elements and d-spacings (Figure 2C). We observed a pseudo-six-fold rotation axis that is best described as 3-fold screw axis, consistent with tbo-topology. Additionally, near atomic-resolution TEM demonstrated that the distance between large diffraction centers was approximately 1.6 nm (16 Å), which is consistent with both tbo models presented later in this manuscript. Additionally, we were able to measure interplanar angles of 82°, which is consistent with the average angle present in HKUST-1 when viewed along the unit cell edges (Figure S10). However, without atomic-resolution TEM, the number of uranium atoms could not be differentiated.
To clarify how many uranium atoms were in the node, we employed thermogravimetric analysis (TGA) to determine weight percentages (Figure S10) of uranium and linker. Previous TGA data of three-dimensional HHTP-based MOFs demonstrate complete conversion to metal-oxide species by 500 °C,29, 30 which we also see in NU-1700. For our analysis, we assumed that the metal-oxide species produced are either UO2 or U3O8, which are the most common and stable uranium oxides. The first mass drop (Figure S11) occurs immediately from 30 to 70 °C, consistent with adsorbed water leaving the framework. The mass continues to drop gradually from 70 °C to 300 °C, with a mass loss of 12 %, attributable to vaporization of DMF and other solvents. Decomposition of the HHTP linker (36 % mass loss) occurs at 300 °C and finishes at approximately 475 °C, wherein the remaining sample mass was 44 % of the original mass. This compares very favorably to the predicted weight percentage of HHTP linker present when considering a paddlewheel-based tbo MOF containing two metals per node, such as HKUST-1, as a model (35 %, Tables S3 and S4). Additionally, the relative weights of solvent, linker, and remaining uranium and their ratio to one another rule out the presence of a U4+ node containing more than two atoms, such as the large U4+ clusters (Figure 1) previously mentioned. We thus assign the linker to node radio as 3 : 2.
Having gathered evidence to support the presence of a tbo topology, we utilized computational modeling to produce a hypothetical model of NU-1700 using the PBE-D3(BJ) level of theory45-47 with a 4.0 eV effective Hubbard U correction48 on the uranium f-orbitals. Following extensive testing of different coordination motifs (Figures S12, S14, and S16), including models with single-ion and dimeric nodes, we determined the coordination of the U to be most in line with a paddlewheel. For a more in-depth discussion involving PXRD matching (Figures S13 and S15) and the DFT modeling used (Table S5), we encourage readers to refer to the Supporting Information. The first paddlewheel model, NU-1700-P1 (Figure S14) contains a four-connected U paddlewheel node and was optimized such that the atomic positions were allowed to relax while retaining the experimentally determined unit cell parameters from PXRD (i.e., 36.904 Å for the conventional cell and 26.095 Å for the primitive cell). The second model, NU-1700-P2 (Figure 3) is a modified version of NU-1700-P1. We used an unrelaxed paddlewheel as the baseline structure and added four H2O molecules to each U site, resulting in each U atom being eight-coordinate with four linkers and four H2O moieties. At this point, we performed a structural relaxation for all H2O molecules present in the solvated paddlewheel, while keeping all other atoms fixed following their initial relaxation. We consider this model to be the most representative of NU-1700, as its PXRD pattern is in good agreement with the experimental data. Furthermore, NU-1700-P2 exhibits characteristics that are more in line with UIV chemistry than those of NU-1700-P1, such as coordination numbers of 8.
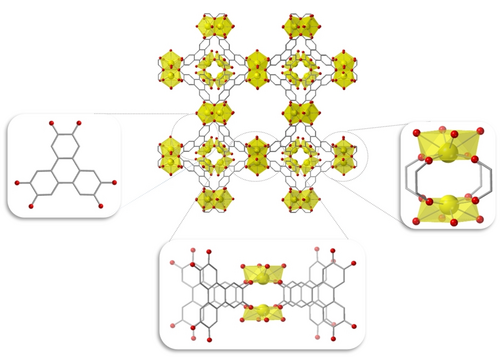
NU-1700-P2 (center) and its building blocks HHTP (left), the truncated paddlewheel node (right) and the extended paddlewheel node (bottom center). Hydrogen atoms are omitted for clarity. Color key: U (yellow), O (red), C (gray).
In the paddlewheel models, the uranium species are predicted to have a high-spin ground state with approximately two unpaired electrons per uranium atom, consistent with a possible 4+ oxidation state (Table S2). In NU-1700-P1 the uranium atoms exhibit a distorted square planar geometry based on the DFT calculations, such that the uranium is slightly puckered relative to the surrounding oxygen atoms. The bonds present within NU-1700-P2 are comparable to those of CeIV and UIV tetrakis(catecholo) species.49, 50
Figure 4 shows a comparison of the PXRD patterns for the DFT-computed model NU-1700-P2 against the as-synthesized and activated form of NU-1700, while Figure S13 shows the comparison of NU-1700-P1 against the as-synthesized and activated forms of NU-1700. Based on the peak positions, the PXRD pattern for NU-1700-P2 is in agreement with that of NU-1700, suggesting that the computational model (with fixed lattice constants determined experimentally) is likely to be representative of the structure of NU-1700 while further supporting the overall solvated paddlewheel-based tbo structure. We note that the powder pattern for activated NU-1700 is slightly shifted compared to its as-synthesized counterpart, potentially due to removal of coordinated solvent inducing some structural distortion. Taken together, we believe NU-1700-P2 is likely to be a plausible model for the framework.
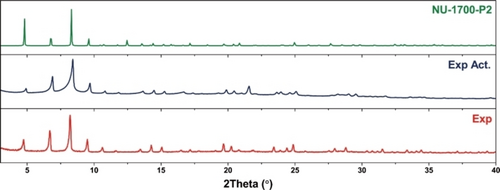
PXRD patterns of as-synthesized NU-1700 (red), activated NU-1700 (dark blue), the model NU-1700-P2 (green).
Conclusion
In this work we have identified a new uranium-catecholate MOF NU-1700 and propose its structure to be based on uranium-paddlewheel nodes connected to HHTP linkers in tbo topology. While the exclusive nano-sizing of the particles did not allow for single crystal X-ray diffraction, we were able to utilize a suite of characterization techniques and computational modeling to provide a plausible structure. This work, in combination with previous literature on Ln-HHTP species,51, 52 demonstrates that catecholate building blocks can induce both unexpected structural characteristics when paired with f-block elements. We hope that this motivates future research on the interactions of non-innocent organic linking species with f-block elements for the creation of novel and highly unusual porous materials, particularly working towards the stabilization of more unusual oxidation states in uranium.
Supporting Information
The authors have cited additional references within the Supporting Information.54-65
Appendix
NU-1700 MOFid/MOFkey:53
-
MOFkey:
-
U.CVDALFASPVBUKU.MOFkey-v1.tbo
-
MOFid:
-
[O]c1cc2c(cc1[O])c1cc([O])c(cc1c1c2cc([O])c(c1)[O])[O].[U][U] MOFid-v1.tbo.cat0
Acknowledgments
J.G.K., M.F., and O.K.F. acknowledge support from the U.S. Department of Energy, National Nuclear Security Administration, under Award Number DE-NA0003763. R.Q.S. acknowledges support from the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences via the Institute for Catalysis in Energy Processes (ICEP) under Award Number DOE DE-FG02-03-ER15457. K.O.K. acknowledges support from the IIN Postdoctoral Fellowship and the Northwestern University International Institute of Nanotechnology. J.G.K. would like to acknowledge the National Science Foundation Graduate Research Fellowship (Grant DGE-2234667.) A.S.R. acknowledges support via a Miller Research Fellowship from the Miller Institute for Basic Research in Science, University of California, Berkeley. X.G. would like to acknowledge the International Institute of Nanotechnology Ryan Fellowship. T.E. and M.S. acknowledge support from the Laboratory Directed Research and Development program at Los Alamos under project number 20180128ER. This work was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under award no. DE-FG02-99ER14999 (M.R.W.). The authors thank the Center for Sustainable Energy at Notre Dame (ND Energy) Materials Characterization Facility for the use of the PHI VersaProbe II X-ray photoelectron spectrometer. This work made use of the IMSERC Crystallography and NMR facilities at Northwestern University, which have received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-2025633), and Northwestern University. This work made use of the Keck-II facility of Northwestern University's NUANCE Center, which has received support from the SHyNE Resource (NSF ECCS-2025633), the IIN, and Northwestern's MRSEC program (NSF DMR-1720139). The authors acknowledge computing support from the Quest high-performance computing facility at Northwestern University. J.G.K. would also like to thank Thomas R. Sheridan for helpful discussion regarding TGA, as well as Dr. Timur Islamoglu for advice regarding activation.
Conflict of interest
Omar K. Farha and Randall Q. Snurr have a financial interest in NuMat Technologies, a company that seeks to commercialize metal-organic frameworks.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.