Total Synthesis of Sculponin U through a Photoinduced Radical Cascade Cyclization
Wei Cao
College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorZhen Wang
College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorYan Hao
College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorProf. Dr. Tianli Wang
College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shaomin Fu
College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Bo Liu
College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorWei Cao
College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorZhen Wang
College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorYan Hao
College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorProf. Dr. Tianli Wang
College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shaomin Fu
College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Bo Liu
College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorGraphical Abstract
Abstract
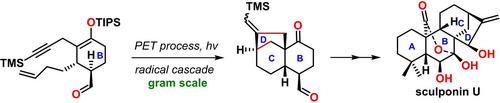
We have accomplished the total synthesis of sculponin U, a polycyclic C-20-oxygenated kaurane diterpenoid featuring a 7,20-lactone-hemiketal bridge, through a radical cascade cyclization triggered by photoinduced electron transfer (PET) of a silyl enolate to form the cyclohexanone-fused bicyclo[3.2.1]octane skeleton. Other key points in our synthetic strategy encompass a Diels–Alder reaction to construct the middle six-membered ring of sculponin U, and an intramolecular radical cyclization induced by iron-catalyzed hydrogen atom transfer to close the western cyclohexane ring. Successful preparation of the enantiopure silyl enolate as the PET precursor enables the asymmetric total synthesis of sculponin U, opening a new avenue for divergent syntheses of structurally related C-20-oxygenated kaurane congeners and pharmaceutical derivatives thereof.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202305516-sup-0001-compound19.cif328.1 KB | Supporting Information |
| anie202305516-sup-0001-compound42.cif341.8 KB | Supporting Information |
| anie202305516-sup-0001-compound47.cif1.4 MB | Supporting Information |
| anie202305516-sup-0001-misc_information.pdf4.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1P. Quitt, E. Mosettig, R. C. Cambie, P. S. Rutledge, L. H. Briggs, J. Am. Chem. Soc. 1961, 83, 3720.
- 2For the related seminal review articles, see:
- 2aH.-D. Sun, S.-X. Huang, Q.-B. Han, Nat. Prod. Rep. 2006, 23, 673;
- 2bP. A. Garcia, A. B. Oliveira, R. Batista, Molecules 2007, 12, 455;
- 2cM. Liu, W.-G. Wang, H.-D. Sun, J.-X. Pu, Nat. Prod. Rep. 2017, 34, 1090.
- 3
- 3aT. Arai, Y. Koyama, T. Suenaga, T. Morita, J. Antibiot. Ser. A 1963, 16, 132;
- 3bI. Kubo, T. Kamikawa, T. Kubota, Tetrahedron 1974, 30, 615;
- 3cI. Kubo, M. Yaniguchi, Y. Satomura, T. Kubota, Agric. Biol. Chem. 1974, 38, 1261;
- 3dT. Arai, Y. Koyama, T. Suenaga, H. Kaji, Chemotherapy 1961, 9, 404;
- 3eE. Fujita, Y. Nagao, K. Kaneko, S. Nakazawa, H. Kuroda, Chem. Pharm. Bull. 1976, 24, 2118;
- 3fE. Blackburn, Mol. Cancer Res. 2005, 3, 477;
- 3gL. Li, G. G. L. Yue, C. B. S. Lau, H. Sun, K. P. Fung, P. C. Leung, Q. Han, P. S. Leung, Toxicol. Appl. Pharmacol. 2012, 262, 80;
- 3hC.-H. Leung, S. P. Grill, W. Lam, W. Cao, H.-D. Sun, Y.-C. Cheng, Mol. Pharmacol. 2006, 70, 1946;
- 3iC.-X. Liu, Q.-Q. Yin, H.-C. Zhou, Y.-L. Wu, J.-X. Pu, L. Xia, W. Liu, X. Huang, T. Jiang, M.-X. Wu, L.-C. He, Y.-X. Zhao, X.-L. Wang, W.-L. Xiao, H.-Z. Chen, Q. Zhao, A.-W. Zhou, L.-S. Wang, H.-D. Sun, G.-Q. Chen, Nat. Chem. Biol. 2012, 8, 486;
- 3jH. Li, B. Sun, M. Wang, X. Hu, X. Gao, S. Xu, Y. Xu, J. Xu, H. Hua, D. Li, Arch. Pharmacal Res. 2018, 41, 1051;
- 3kM. S. Sarwar, Y.-X. Xia, Z.-M. Liang, S. W. Tsang, H.-J. Zhang, Biomol. Eng. 2020, 10, 144;
- 3lY. Sun, Y. Qiao, Y. Liu, J. Zhou, X. Wang, H. Zheng, Z. Xu, J. Zhang, Y. Zhou, L. Qian, C. Zhang, H. Lou, Redox. Biol. 2021, 43, 101977.
- 4For the related seminal review articles, see:
- 4aK. E. Lazarski, B. J. Morita, R. J. Thomson, Angew. Chem. Int. Ed. 2014, 53, 10588;
- 4bL. Zhu, S.-H. Huang, J. Yu, R. Hong, Tetrahedron Lett. 2015, 56, 23;
- 4cP. S. Riehl, Y. C. DePorre, A. M. Armaly, E. J. Groso, C. S. Schindler, Tetrahedron 2015, 71, 6629;
- 4dM. Du, X. Lei, Chin. J. Org. Chem. 2015, 35, 2447;
- 4eX. Zhao, B. Cacherat, Q. Hu, D. Ma, Nat. Prod. Rep. 2022, 39, 119.
- 5
- 5aR. A. Bell, R. E. Ireland, R. A. Partyka, J. Org. Chem. 1966, 31, 2530;
- 5bK. Mori, M. Matsui, Tetrahedron Lett. 1966, 7, 175;
10.1016/S0040-4039(00)70209-7 Google Scholar
- 5cK. Mori, M. Matsui, N. Ikekawa, Y. Sumiki, Tetrahedron Lett. 1966, 7, 3395;
10.1016/S0040-4039(01)82800-8 Google Scholar
- 5dK. Mori, M. Matsui, Tetrahedron 1968, 24, 3095;
- 5eK. Mori, Y. Nakahara, M. Matsui, Tetrahedron Lett. 1970, 11, 2411;
10.1016/S0040-4039(01)98242-5 Google Scholar
- 5fK. Mori, Y. Nakahara, M. Matsui, Tetrahedron 1972, 28, 3217;
- 5gF. E. Ziegler, J. A. Kloek, Tetrahedron 1977, 33, 373;
- 5hL. A. Paquette, D. Backhaus, R. Braun, J. Am. Chem. Soc. 1996, 118, 11990;
- 5iB. B. Snider, J. Y. Kiselgof, B. M. Foxman, J. Org. Chem. 1998, 63, 7945;
- 5jM. Toyota, T. Wada, M. Ihara, J. Org. Chem. 2000, 65, 4565;
- 5kB. B. Snider, Tetrahedron 2009, 65, 10738;
- 5lJ. Y. Cha, J. T. S. Yeoman, S. E. Reisman, J. Am. Chem. Soc. 2011, 133, 14964;
- 5mE. C. Cherney, J. C. Green, P. S. Baran, Angew. Chem. Int. Ed. 2013, 52, 9019;
- 5nL. Zhu, R. Hong, Chin. J. Chem. 2013, 31, 111;
- 5oL. Zhu, J. Luo, R. Hong, Org. Lett. 2014, 16, 2162;
- 5pW. Liu, H. Li, P.-J. Cai, Z. Wang, Z.-X. Yu, X. Lei, Angew. Chem. Int. Ed. 2016, 55, 3112;
- 5qX. Zhao, W. Li, J. Wang, D. Ma, J. Am. Chem. Soc. 2017, 139, 2932;
- 5rC. He, J.-L. Hu, Y.-B. Wu, H.-F. Ding, J. Am. Chem. Soc. 2017, 139, 6098;
- 5sJ. Wang, D. Ma, Angew. Chem. Int. Ed. 2019, 58, 15731;
- 5tB. Hong, W. Liu, J. Wang, J. Wu, Y. Kadonaga, P.-J. Cai, H.-X. Lou, Z.-X. Yu, H. Li, X. Lei, Chem 2019, 5, 1671;
- 5uJ. Wu, Y. Kadonaga, B. Hong, J. Wang, X. Lei, Angew. Chem. Int. Ed. 2019, 58, 10879;
- 5vJ. Wang, B. Hong, D. Hu, Y. Kadonaga, R. Tang, X. Lei, J. Am. Chem. Soc. 2020, 142, 2238;
- 5wY. Que, H. Shao, H. He, S. Gao, Angew. Chem. Int. Ed. 2020, 59, 7444;
- 5xJ. Guo, B. Li, W. Ma, M. Pitchakuntla, Y. Jia, Angew. Chem. Int. Ed. 2020, 59, 15195;
- 5yZ. Xu, Y. Zong, Y. Qiao, J. Zhang, X. Liu, M. Zhu, Y. Xu, H. Zheng, L. Fang, X. Wang, H. Lou, Angew. Chem. Int. Ed. 2020, 59, 19919;
- 5zJ. Zhuo, C. Zhu, J. Wu, Z. Li, C. Li, J. Am. Chem. Soc. 2022, 144, 99;
- 5aaX.-H. Zhao, L.-L. Meng, X.-T. Liu, P.-F. Shu, C. Yuan, X.-T. An, T.-X. Jia, Q.-Q. Yang, X. Zhen, C.-A. Fan, J. Am. Chem. Soc. 2022, 144, 311;
- 5abT. Suzuki, W. Ikeda, A. Kanno, K. Ikeuchi, K. Tanino, Chem. Eur. J. 2023, 29, e202203511;
- 5acX.-H. Zhao, L.-L. Meng, X.-T. Liu, P.-F. Shu, C. Yuan, X.-T. An, T.-X. Jia, Q.-Q. Yang, X. Zhen, C.-An. Fan, J. Am. Chem. Soc. 2023, 145, 311.
- 6For synthesis of seco-kaurane diterpenoids, see:
- 6aE. Fujita, M. Shibuya, S. Nakamura, Y. Okada, T. Fujita, J. Chem. Soc. Chem. Commun. 1972, 1107;
- 6bE. Fujita, M. Shibuya, S. Nakamura, Y. Okada, T. Fujita, J. Chem. Soc. Chem. Commun. 1974, 165;
- 6cM. J. Kenny, L. N. Mander, S. P. Sethi, Tetrahedron Lett. 1986, 27, 3923;
- 6dM. J. Kenny, L. N. Mander, S. P. Sethi, Tetrahedron Lett. 1986, 27, 3927;
- 6eL. A. Paquette, D. Backhaus, R. Braun, T. L. Underiner, K. Fuchs, J. Am. Chem. Soc. 1997, 119, 9662;
- 6fG. Adamson, L. N. Mander, Aust. J. Chem. 2003, 56, 805;
- 6gJ. Gong, G. Lin, W. Sun, C.-C. Li, Z. Yang, J. Am. Chem. Soc. 2010, 132, 16745;
- 6hF. Peng, S. J. Danishefsky, J. Am. Chem. Soc. 2012, 134, 18860;
- 6iP. Lu, Z. Gu, A. Zakarian, J. Am. Chem. Soc. 2013, 135, 14552;
- 6jP. Lu, A. Mailyan, D. M. Guptil, H. Wang, H. M. L. Davies, A. Zakarian, J. Am. Chem. Soc. 2014, 136, 17738;
- 6kC. Zheng, I. Dubovyk, K. E. Lazarski, R. J. Thomson, J. Am. Chem. Soc. 2014, 136, 17750;
- 6lZ. Pan, C. Zheng, H. Wang, Y. Li, B. Cheng, H. Zhai, Org. Lett. 2014, 16, 216;
- 6mB. J. Moritz, D. J. Mack, L. Tong, R. J. Thomson, Angew. Chem. Int. Ed. 2014, 53, 2988;
- 6nA. Cernijenko, R. Risgaard, P. S. Baran, J. Am. Chem. Soc. 2016, 138, 9425;
- 6oZ. Lv, B. Chen, C. Zhang, G. Liang, Chem. Eur. J. 2018, 24, 9773;
- 6pS. Pan, S. Chen, G. Dong, Angew. Chem. Int. Ed. 2018, 57, 6333;
- 6qJ.-P. Zhang, Z.-J. Li, J.-M. Zhuo, Y. Cui, T. Han, C. Li, J. Am. Chem. Soc. 2019, 141, 8372.
- 7For synthesis of C-20 oxygenated kaurane diterpenoids, see:
- 7aW. S. Zhou, Y. X. Cheng, Sci. China Ser. B 1992, 35, 194;
- 7bK. Mori, S. Aki, Liebigs Ann. Chem. 1993, 97;
- 7cE. J. Corey, K. Liu, J. Am. Chem. Soc. 1997, 119, 9929;
- 7dJ. T. S. Yeoman, V. W. Mak, S. E. Reisman, J. Am. Chem. Soc. 2013, 135, 11764;
- 7eS. Kobayashi, K. Shibukawa, Y. Hamada, T. Kuruma, A. Kawabata, A. Masuyama, J. Org. Chem. 2018, 83, 1606;
- 7fF. Su, Y.-D. Lu, L.-R. Kong, J.-J. Liu, T.-P. Luo, Angew. Chem. Int. Ed. 2018, 57, 760;
- 7gL.-Z. Zhu, W.-J. Ma, M.-X. Zhang, M. M.-L. Lee, W.-Y. Wong, B. D. Chan, Q.-Q. Yang, W.-T. Wong, W. C.-S. Tai, C.-S. Lee, Nat. Commun. 2018, 9, 1283;
- 7hL. Kong, F. Su, H. Yu, Z. Jiang, Y. Lu, T. Luo, J. Am. Chem. Soc. 2019, 141, 20048;
- 7iJ. Ao, C. Sun, B. Chen, N. Yu, G. Liang, Angew. Chem. Int. Ed. 2022, 61, e202114489.
- 8S. Fu, B. Liu, Chin. J. Chem. 2022, 40, 407.
- 9H.-Y. Jiang, W.-G. Wang, M. Zhou, H.-Y. Wu, R. Zhan, X.-N. Li, X. Du, Y. Li, J.-X. Pu, H.-D. Sun, Fitoterapia 2014, 93, 142.
- 10
- 10aA. Heidbreder, J. Mattay, Tetrahedron Lett. 1992, 33, 1973;
- 10bE. Albrecht, J. Averdung, E. W. Bischof, A. Heidbreder, T. Kirschberg, F. Műller, J. Mattay, J. Photochem. Photobiol. A 1994, 82, 219;
- 10cS. Hintz, R. Fröhlich, J. Mattay, Tetrahedron Lett. 1996, 37, 7349;
- 10dS. Hintz, J. Mattay, R. van Eldik, W.-F. Fu, Eur. J. Org. Chem. 1998, 1583;
10.1002/(SICI)1099-0690(199808)1998:8<1583::AID-EJOC1583>3.0.CO;2-R CAS Web of Science® Google Scholar
- 10eL. Ackermann, A. Heidbreder, F. Wurche, F.-G. Klärner, J. Mattay, J. Chem. Soc. Perkin Trans. 2 1999, 863;
- 10fJ. O. Bunte, S. Rinne, J. Mattay, Synthesis 2004, 619;
- 10gJ. O. Bunte, E. K. Heilmann, B. Hein, J. Mattay, Eur. J. Org. Chem. 2004, 3535;
- 10hH. Rinderhagen, J. Mattay, Eur. J. Chem. 2004, 10, 851;
- 10iP. A. Waske, N. T. Tzvetkov, J. Mattay, Synlett 2007, 669.
- 11
- 11aM. S. Galliher, B. J. Roldan, C. R. J. Stephenson, Chem. Soc. Rev. 2021, 50, 10044;
- 11bK. Gennaiou, A. Kelesidis, M. Kourgiantaki, A. L. Zografos, Beilstein J. Org. Chem. 2023, 19, 1.
- 12See Supporting Information for detail.
- 13
- 13aN. A. Romero, D. A. Nicewicz, Chem. Rev. 2016, 116, 10075;
- 13bM. V. Bobo, J. J. Kuchta III, A. K. Vannucci, Org. Biomol. Chem. 2021, 19, 4816;
- 13cJ. Xu, J. Cao, X. Wu, H. Wang, X. Yang, X. Tang, R. W. Toh, R. Zhou, E. K. L. Yeow, J. Wu, J. Am. Chem. Soc. 2021, 143, 13266.
- 14I. R. Gould, D. Ege, J. E. Moser, S. Farid, J. Am. Chem. Soc. 1990, 112, 4290.
- 15S. R. Magnuson, L. Sepp-Lorenzino, N. Rosen, S. J. Danishefsky, J. Am. Chem. Soc. 1998, 120, 1615.
- 16For seminal review articles on HAT-promoted natural product synthesis, see:
- 16aJ. Wu, Z. Ma, Org. Chem. Front. 2021, 8, 7050;
- 16bS. A. Green, S. W. M. Crossley, J. L. M. Matos, S. Vásquez-Céspedes, S. L. Shevick, R. A. Shenvi, Acc. Chem. Res. 2018, 51, 2628. For selected research works, see:
- 16cJ. C. Lo, J. Gui, Y. Yabe, C.-M. Pan, P. S. Baran, Nature 2014, 516, 343;
- 16dJ. C. Lo, Y. Yabe, P. S. Baran, J. Am. Chem. Soc. 2014, 136, 1304.
- 17Deposition Numbers 2239652 (for 19′), 2239650 (for 42′), and 2253497 (for 47) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.