A Supported Catalyst that Enables the Synthesis of Colorless CO2-Polyols with Ultra-Low Molecular Weight
Qingxian Kuang
Key Laboratory of Polymer Ecomaterial, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022 China
University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorRuoyu Zhang
Key Laboratory of Polymer Ecomaterial, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022 China
University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorZhenzhen Zhou
Key Laboratory of Polymer Ecomaterial, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022 China
University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCan Liao
Key Laboratory of Polymer Ecomaterial, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022 China
University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shunjie Liu
Key Laboratory of Polymer Ecomaterial, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022 China
University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorProf. Dr. Xuesi Chen
Key Laboratory of Polymer Ecomaterial, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022 China
University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xianhong Wang
Key Laboratory of Polymer Ecomaterial, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022 China
University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorQingxian Kuang
Key Laboratory of Polymer Ecomaterial, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022 China
University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorRuoyu Zhang
Key Laboratory of Polymer Ecomaterial, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022 China
University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorZhenzhen Zhou
Key Laboratory of Polymer Ecomaterial, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022 China
University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCan Liao
Key Laboratory of Polymer Ecomaterial, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022 China
University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shunjie Liu
Key Laboratory of Polymer Ecomaterial, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022 China
University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorProf. Dr. Xuesi Chen
Key Laboratory of Polymer Ecomaterial, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022 China
University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xianhong Wang
Key Laboratory of Polymer Ecomaterial, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022 China
University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorGraphical Abstract
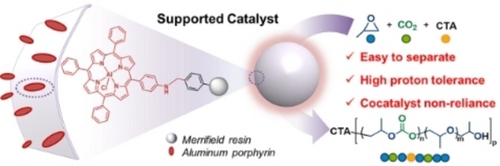
A strategy for constructing a supported catalyst featuring immobilization-enhanced proton tolerance for the synthesis of colorless ULMW CO2-polyols is proposed. The key design feature lies in the loading of aluminum aminoporphyrins on swellable Merrifield resin to afford the multiple cooperativity of porphyrins in the supported catalysts.
Abstract
Ultra-low molecular weight (ULMW) CO2-polyols with well-defined hydroxyl end groups represent useful soft segments for the preparation of high-performance polyurethane foams. However, owing to the poor proton tolerance of catalysts towards CO2/epoxide telomerization, it remains challenging to synthesize ULMW yet colorless CO2-polyols. Herein, we propose an immobilization strategy of constructing supported catalysts by chemical anchoring of aluminum porphyrin on Merrifield resin. The resulting supported catalyst displays both extremely high proton tolerance (≈8000 times the equivalents of metal centers) and independence of cocatalyst, affording CO2-polyols with ULMW (580 g mol−1) and high polymer selectivity (>99 %). Moreover, the ULMW CO2-polyols with various architectures (tri-, quadra-, and hexa-arm) can be obtained, suggesting the wide proton universality of supported catalysts. Notably, benefiting from the heterogeneous nature of the supported catalyst, colorless products can be facilely achieved by simple filtration. The present strategy provides a platform for the synthesis of colorless ULMW polyols derived from not only CO2/epoxides, but also lactone, anhydrides etc. or their combinations.
Conflict of interest
The authors declare no competing financial interests.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202305186-sup-0001-misc_information.pdf1.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1V. K. Kensy, R. L. Tritt, F. M. Haque, L. M. Murphy, D. B. J. Knorr, S. M. Grayson, A. J. Boydston, Angew. Chem. Int. Ed. 2020, 59, 9074–9079.
- 2
- 2aT. W. Ching, J. Zhang, D. Collias, J. McDaniel, A. Simonyan, A. Tanksale, M. M. Banaszak Holl, ACS Sustainable Chem. Eng. 2020, 8, 14504–14510;
- 2bP. de Sainte Claire, Macromolecules 2009, 42, 3469–3482;
- 2cK. Fukatsu, S. Kokot, Polym. Degrad. Stab. 2001, 72, 353–359;
- 2dG. I. Peterson, K. T. Bang, T. L. Choi, J. Am. Chem. Soc. 2018, 140, 8599–8608;
- 2eX. Xu, T. Liu, Y. Chai, M. Kong, Y. Luo, Fine Chem. 2019, 36, 2213–2219;
- 2fA. L. Kocen, S. Cui, T. W. Lin, A. M. LaPointe, G. W. Coates, J. Am. Chem. Soc. 2022, 144, 12613–12618.
- 3P. Cui, C. T. Song, X. H. Zhang, D. Chen, Y. H. Ma, W. T. Yang, Chin. J. Polym. Sci. 2019, 37, 646–653.
- 4
- 4aC. M. R. Abreu, P. V. Mendonça, A. C. Serra, J. F. J. Coelho, A. V. Popov, G. Gryn'ova, M. L. Coote, T. Guliashvili, Macromolecules 2012, 45, 2200–2208;
- 4bY. Zhang, R. J. Keaton, L. R. Sita, J. Am. Chem. Soc. 2003, 125, 9062–9069;
- 4cJ. Wang, K. Matyjaszewski, J. Am. Chem. Soc. 1995, 117, 5614–5615;
- 4dW. A. Braunecker, K. Matyjaszewski, Prog. Polym. Sci. 2007, 32, 93–146.
- 5
- 5aN. Ajellal, J. F. Carpentier, C. Guillaume, S. M. Guillaume, M. Helou, V. Poirier, Y. Sarazina, A. Trifonovb, Dalton Trans. 2010, 39, 8363–8376;
- 5bC. Y. Hu, X. Pang, X. S. Chen, Macromolecules 2022, 55, 1879–1893;
- 5cS. Paul, Y. Zhu, C. Romain, R. Brooks, P. K. Saini, C. K. Williams, Chem. Commun. 2015, 51, 6459–6479;
- 5dM. J. Sanford, N. J. Van Zee, G. W. Coates, Chem. Sci. 2018, 9, 134–142;
- 5eS. Inoue, J. Polym. Sci. Part A 2000, 38, 2861–2871.
- 6
- 6aJ. Langanke, A. Wolf, J. Hofmann, K. Böhm, M. A. Subhani, T. E. Müller, W. Leitner, C. Gürtler, Green Chem. 2014, 16, 1865–1870;
- 6bR. Gong, H. Cao, H. M. Zhang, L. J. Qiao, F. S. Wang, X. H. Wang, Macromolecules 2020, 53, 6322–6330;
- 6cY. G. Gao, Y. S. Qin, X. J. Zhao, F. S. Wang, X. H. Wang, J. Polym. Res. 2012, 19, 9878–9886.
- 7M. R. Kember, C. K. Williams, J. Am. Chem. Soc. 2012, 134, 15676–15679.
- 8D. J. Darensbourg, G. P. Wu, Angew. Chem. Int. Ed. 2013, 52, 10602–10606.
- 9A. C. Deacy, E. Moreby, A. Phanopoulos, C. K. Williams, J. Am. Chem. Soc. 2020, 142, 19150–19160.
- 10A. Cyriac, S. H. Lee, J. K. Varghese, E. S. Park, J. H. Park, B. Y. Lee, Macromolecules 2010, 43, 7398–7401.
- 11
- 11aH. Cao, R. Y. Zhang, Z. Z. Zhou, S. J. Liu, Y. H. Tao, F. S. Wang, X. H. Wang, ACS Catal. 2022, 12, 481–490;
- 11bH. Cao, R. N. Gong, Z. Z. Zhou, X. H. Wang, F. S. Wang, Acta. Polym. Sin. 2021, 8, 1006–1014;
- 11cC. W. Zhuo, H. Cao, X. S. Wang, S. J. Liu, X. H. Wang, Chin. Chem. Lett. 2022, https://doi.org/10.1016/j.cclet.2022.108011.
- 12V. S. Shende, V. B. Saptal, B. M. Bhanage, Chem. Rec. 2019, 19, 2022–2043.
- 13
- 13aX. H. Zhang, R. J. Wei, Y. Y. Zhang, B. Y. Du, Z. Q. Fan, Macromolecules 2015, 48, 536–544;
- 13bP. Wang, S. j. Liu, Y. S. Qin, X. H. Wang, F. S. Wang, Acta. Polym. Sin. 2017, 2, 259–265;
- 13cS. j. Liu, Y. S. Qin, X. S. Chen, X. H. Wang, F. S. Wang, Polym. Chem. 2014, 5, 6171–6179;
- 13dY. G. Gao, L. Gu, Y. S. Qin, X. H. Wang, F. S. Wang, J. Polym. Sci. Part A 2012, 50, 5177–5184.
- 14C. Chen, Y. Gnanou, X. S. Feng, Macromolecules 2023, 56, 892–898.
- 15
- 15aC. Copéret, F. Allouche, K. W. Chan, M. P. Conley, M. F. Delley, A. Fedorov, I. B. Moroz, V. Mougel, M. Pucino, K. Searles, K. Yamamoto, P. A. Zhizhko, Angew. Chem. Int. Ed. 2018, 57, 6398–6440;
- 15bT. R. Boussie, C. Coutard, H. Turner, V. Murphy, T. S. Powers, Angew. Chem. Int. Ed. 1998, 37, 3272–3275;
10.1002/(SICI)1521-3773(19981217)37:23<3272::AID-ANIE3272>3.0.CO;2-R CAS PubMed Web of Science® Google Scholar
- 15cC. Zou, G. F. Si, C. L. Chen, Nat. Commun. 2022, 13, 1954.
- 16
- 16aJ. P. Collman, P. S. Wagenknecht, J. E. Hutchison, Angew. Chem. Int. Ed. Engl. 1994, 33, 1537–1554;
- 16bH. Sugimoto, K. Kuroda, Macromolecules 2008, 41, 312–317;
- 16cC. Chatterjee, M. H. Chisholm, Inorg. Chem. 2011, 50, 4481–4492;
- 16dH. Cao, Y. S. Qin, C. W. Zhuo, X. H. Wang, F. S. Wang, ACS Catal. 2019, 9, 8669–8676;
- 16eR. Y. Zhang, Q. X. Kuang, H. Cao, S. J. Liu, X. S. Chen, X. H. Wang, F. S. Wang, CCS Chem. 2023, 5, 750–760.
- 17C. E. Anderson, S. I. Vagin, W. Xia, H. Jin, B. Rieger, Macromolecules 2012, 45, 6840–6849.
- 18
- 18aC. L. Ren, X. Zhu, N. Zhao, Y. Shen, L. F. Chen, S. F. Liu, Z. B. Li, Eur. Polym. J. 2019, 119, 130–135;
- 18bJ. Lu, P. H. Toy, Chem. Rev. 2009, 109, 815–838.
- 19
- 19aQ. Hao, Y. Tao, X. Ding, Y. Yang, J. Feng, R. L. Wang, X. M. Chen, G. L. Chen, X. Li, H. O. Yang, X. Hu, J. Tian, B. H. Han, G. Zhu, W. Wang, F. Zhang, B. Tan, Z. T. Li, D. Wang, L. J. Wan, Sci. China Chem. 2023, 66, 620–682;
- 19bF. Zaera, Chem. Rev. 2022, 122, 8594–8757.
- 20
- 20aT. Ohkawara, K. Suzuki, K. Nakano, S. Mori, K. Nozaki, J. Am. Chem. Soc. 2014, 136, 10728–10735;
- 20bD. R. Moore, M. Cheng, E. B. Lobkovsky, G. W. Coates, J. Am. Chem. Soc. 2003, 125, 11911–11924;
- 20cM. W. Lehenmeier, S. Kissling, P. T. Altenbuchner, C. Bruckmeier, P. Deglmann, A. K. Brym, B. Rieger, Angew. Chem. Int. Ed. 2013, 52, 9821–9826;
- 20dJ. Liu, W. M. Ren, Y. Liu, X. B. Lu, Macromolecules 2013, 46, 1343–1349;
- 20eG. W. Coates, D. R. Moore, Angew. Chem. Int. Ed. 2004, 43, 6618–6639;
- 20fF. Jutz, A. Buchard, M. R. Kember, S. B. Fredriksen, C. K. Williams, J. Am. Chem. Soc. 2011, 133, 17395–17405.
- 21M. Zhao, S. Zhu, G. Zhang, Y. Wang, Y. Liao, J. Xu, X. Zhou, X. Xie, Macromolecules 2023, 56, 2379–2387.