Solar Light Driven H2O2 Production and Selective Oxidations Using a Covalent Organic Framework Photocatalyst Prepared by a Multicomponent Reaction
Graphical Abstract
A covalent organic framework (COF) prepared via the multicomponent Povarov reaction shows a high and long-term stable activity for photocatalytic H2O2 production and size-selective photocatalytic oxidation. The COF can be further used as photocatalyst for a range of important aerobic oxidation reactions including the conversion of alcohols to aldehydes, amines to imines and sulfides to sulfoxides.
Abstract
A chemically stable 2D microporous COF (PMCR-1) was synthesized via the multicomponent Povarov reaction. PMCR-1 exhibits a remarkable and long-term stable photocatalytic H2O2 production rate (60 h) from pure and sea water under visible light. The H2O2 production is markedly enhanced when benzyl alcohol (BA) is added as reductant, which is also due to a strong π–π interaction of BA with dangling phenyl moieties in the COF pores introduced by the multicomponent Povarov reaction. Motivated by the concomitant BA oxidation to benzaldehyde during H2O2 formation, the photocatalytic oxidation of various organic substrates such as benzyl amine and methyl sulfide derivatives was investigated. It is shown that the well-defined micropores of PMCR-1 enable size-selective photocatalytic oxidation.
Introduction
Hydrogen peroxide (H2O2) is one of the most important oxidants and widely utilized for many relevant applications in wastewater treatment, paper bleaching, disinfection, chemical synthesis and also in fuel cells.1-4 This has increased its global annual demand to currently 4 million tons and to estimated 5.7 million tons by 2027.5, 6 Furthermore, due to the comparable energy density between compressed hydrogen and aqueous H2O2 along with the facile storage and transportation of H2O2, it has become an alternative source to store energy. Currently, the anthraquinone (AQ) process is mainly utilized for industrial H2O2 production involving the hydrogenation of AQ over Pd and subsequent oxidation reaction with O2. This process is energy intensive and produces hazardous waste.7 As an alternative, H2O2 can be synthesized directly from H2/O2 mixtures using Pd or Au−Pd metal catalysts which however requires several safety measures.8 For a sustainable, eco-friendly and cost-efficient H2O2 production, alternative technologies are thus demanded. In this context, photocatalytic H2O2 production from water and oxygen through molecular oxygen reduction has become a sustainable alternative.9-12 In this process light irradiation triggers the generation of oxygen-containing intermediates such as superoxide (⋅O2−), singlet oxygen (1O2) or hydroxyl radicals (⋅OH) summarized as reactive oxygen species (ROS).13 These ROS act as vital intermediates in various chemical and environmental applications such as degradation of toxic pollutants and chemical oxidation reactions.14, 15 Therefore, the development of efficient photocatalysts for ROS production is required for several chemical processes.
Organic semiconductors with tunable optical and electronic properties are promising photocatalysts for solar-driven H2O2 production. Materials like carbon nitrides,16-20 linear conjugated polymers,21 conjugated microporous polymers,22, 23 and covalent triazine frameworks24-26 have been investigated for photocatalytic H2O2 generation. In recent years, significant advances have been made to develop entirely organic, crystalline materials formed by covalent linkages via reversible reactions, named covalent organic frameworks (COFs).27, 28 Recently, several COFs have been reported for photocatalytic H2 evolution29-33 and also a few for H2O2 generation.34-38 However, their often-low stability in water, especially at higher or lower pH values, make their applicability for long-term photocatalytic processes challenging. To develop chemically robust COFs, an intriguing linkage chemistry has been developed recently including reversible C−C-binding reactions,39-41 post-synthetic modification42, 44 and multi-component reactions (MCRs).36, 45-51 The latter MCR process enables a facile synthetic approach by combining multiple components in one pot to form robust aromatic linkages and thus chemically and temperature stable COFs.
Beside hydrogen and hydrogen peroxide production, COFs have also been applied as photocatalysts for organic transformation reactions.51-56 To synthesize diverse fine chemicals, selective oxidation of both aliphatic and aromatic compounds are elemental reactions. The conventional oxidation processes carried out industrially employ harsh conditions and hazardous oxidants, yielding production of waste byproducts and increased operational cost with drastic impact on the environment. Therefore, the use of stable and recyclable photoactive COFs for the synthesis of value-added organic products is also demanded for a sustainable production of chemicals.
In this work, the one-pot synthesis of COFs by using the multicomponent Povarov reaction (PMCR-1) is reported. This process yields a novel quinoline-linked microporous MCR COF with phenyl moieties pointing into the pores. PMCR-1 produces high amounts of H2O2 in both water and sea water under natural sunlight over a long period of time (60 h) without loss in catalytic activity. When benzyl alcohol (BA) is added as sacrificial agent, the production of H2O2 is markedly increased together with a selective oxidation of BA to the corresponding aldehyde. Such a selective oxidation is also achieved with other organic compounds such as amines and sulfides. Furthermore, a size-dependent substrate-selectivity in the aerobic oxidation process is observed.
Results and Discussion
A MCR-COF with quinoline linkages and phenyl groups pointing into the pores was synthesized via the three-component one-pot Povarov reaction, which involves aldehyde, amine and substituted vinyl compounds (Figure 1A). The suggested reaction course is verified using similar conditions with molecular model compounds with 3,4-dimethoxy aniline, salicylaldehyde and styrene (Scheme S1 and Figures S1–S2). PMCR-1 was synthesized via the three-component reaction, using 2,4,6-Tris(4-aminophenyl)triazine (TAT), 4,4′,4′′-(1,3,5-triazine-2,4,6-triyl)tribenzaldehyde (TTA) and styrene in a solvent mixture of o-dichlorobenzene/n-butanol (1 : 1), in presence of BF3⋅Et2O, AcOH (6 M) and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) at 120 °C for 72 h. The 4-phenyl-quinoline-linked COF was collected as a golden yellow colored powder in 95 % yield (Figure 1 and Scheme S2).
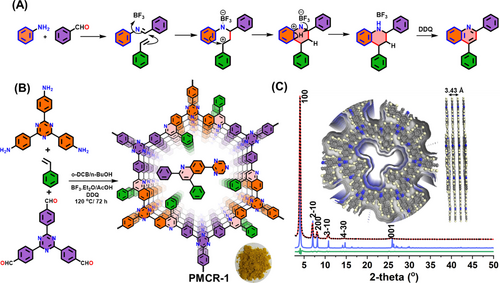
Synthesis, structure and characterization of PMCR-1: (A) Mechanism of the Povarov reaction; (B) Synthesis Scheme for PMCR-1; (C) PXRD pattern for PMCR-1: experimental (black dotted line) and Pawley refined (red line) patterns and corresponding difference plot (green line). The theoretical PXRD pattern for an eclipsed AA stacking model of PMCR-1 is provided as blue line. Top and side view of AA stacking in PMCR-1. (Color code: C, gray spheres; O, red spheres; N, blue spheres, H, pale yellow spheres).
The crystallinity of PMCR-1 was confirmed by powder X-ray diffraction (PXRD) analyses. PMCR-1 exhibits an intense reflection in the low-angle region at 4.0 degrees 2θ (Figure 1C). An additional set of lower symmetry reflections that could not be assigned to the starting monomers indicated the formation of a crystalline framework. The experimental pattern showed a similar set of reflections (Figure 1C), suggesting the formation of the expected 2D layered structure. Hexagonal layered structural models with hcb topology and with different stacking sequences were constructed and geometrically optimized. Comparison of the theoretical with the experimental PXRD pattern suggested the formation of 2D hexagonal layers with an eclipsed AA stacking arrangement in the c direction (Figure S3). Pawley fitting over the full profile was carried out to refine the final unit cell parameters (Table S1). PMCR-1 was fully characterized by solid-state cross-polarization magic-angle-spinning (CP-MAS) 13C NMR and Fourier transform infrared (FTIR), and X-ray photoelectron spectroscopy (XPS). The CP-MAS 13C NMR spectrum of PMCR-1 revealed the generation of new signals characteristic for quinoline groups at 156 and 149 ppm which is well corroborated by the 13C NMR spectrum of the model monomer, where these peaks appear at 155.9 and 148.6 ppm, respectively (Figure 2A and S1).
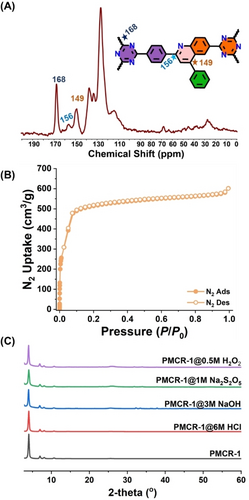
(A) Solid-state 13C NMR spectrum for PMCR-1; (B) N2 sorption at 77 K; (C) Chemical stability of PMCR-1 after 4 days treatment with 6 M HCl, 3 M NaOH, 1 M Na2S2O5 and 0.5 M H2O2.
Also, the signals that can be assigned to the benzene ring pointing into the pores are observed in the aromatic region (115–130 ppm) of the 13C NMR spectrum. The presence of the strong characteristic peaks at 1579 cm−1 in the FTIR spectrum corresponding to pyridyl stretching frequencies from quinoline of PMCR-1 (Figure S4) also confirmed the formation of the expected heterocyclic aromatic linkage. XPS spectra further validated the formation of quinoline linkages in PMCR-1 with the characteristic peak of N 1s present at 400.1 eV (quinoline N) (Figure S5).36, 46 The porosity of PMCR-1 was investigated by N2 sorption experiments at 77 K. N2 sorption displayed a type-I sorption isotherm from which an apparent BET surface area of 1756 m2 g−1 was calculated. The measured value suggests that PMCR-1 reaches its maximum theoretical uptake which is calculated to be 1739 m2 g−1 (Figure 2B). The pore size of PMCR-1 was found to be 7.0 Å and 10.0–14.0 Å using DFT method which is well correlated with the simulated structure (Figure S6). From the TGA profile a minimal weight loss is observed until a temperature of 500 °C measured at N2 atmosphere (Figure S7). The chemical stability was determined by immersing PMCR-1 in 6 M HCl, 3 M NaOH, 1 M Na2S2O5 and 0.5 M H2O2 for 4 days and PXRD analyses of the recovered samples. Quantitative recovery of PMCR-1 and no sign of a loss in crystallinity in the collected PXRD patterns confirmed the high chemical stability of the synthesized COF (Figure 2C).
Field emission scanning electron microscopy (FESEM) image of PMCR-1 showed the formation of branched rod-like structures from aggregated crystallites (Figure S8). Transmission electron microscopy (TEM) images confirmed these aggregated structures while the high-resolution TEM (HRTEM) image displayed crystalline lattice fringes (Figure S9).
The UV/Vis reflectance spectra of PMCR-1 displayed an absorption starting at approx. 475 nm with a maximum at 400 nm (Figure S10). Based on solid-state UV/Vis and valence band (VB) XPS, the VB and CB of PMCR-1 was calculated to be −6.49 and −3.78 eV vs. vacuum, respectively, which is consistent with the calculated HOMO and LUMO energy obtained from DFT calculation (Figures S11–S13).57 The calculated frontier orbitals of PMCR-1 indicate that triazine groups and phenyl quinoline moieties act as electron-withdrawing and electron-donating centers, respectively. To prove the charge separation efficiency, photocurrent and solid-state electron paramagnetic resonance (EPR) CB spectra (g=2.005) were measured in the dark and after illumination with visible light. Both photocurrent and solid-state EPR displayed a significant intensity increase when the light is switched on indicating the efficient photogeneration of charge carriers via the electron transfer from the VB to the CB (Figure 3A and 3B). Also, from the band position it can be anticipated that the reduction of O2 to ⋅O2− and oxidation of H2O to H2O2 should be possible because their redox potentials are in between the CB and VB levels of PMCR-1. Consequently, photocatalytic H2O2 production was tested using PMCR-1 as photocatalyst (10 mg of COF in 22 mL water or 10 : 1 water:alcohol mixtures, measured after 1 h at 25 °C and λ>420 nm, O2 purge 10 min, see Supporting Information for details, Figure S14). A H2O2 production of 1294 μmol h−1 g−1 was observed in pure water and air, which can be slightly increased (1445 μmol h−1 g−1) when pure oxygen is used (Figure 3C). When sacrificial hole scavengers like ethanol or isopropanol (IPA) were added (10 : 1, v/v water/alcohol), the production of H2O2 increased. This can be due to the more efficient hole utilization of the added reductants and also to a better dispersion of the rather hydrophobic COF, with its pendant phenyl groups. PMCR-1 showed H2O2 generation rates of 1941 and 2265 μmol h−1 g−1 using ethanol and IPA as sacrificial agents, respectively. However, when benzyl alcohol (BA) is used, H2O2 production by PMCR-1 is markedly increased (5500 μmol h−1 g−1) (Figure 3C). It can be assumed that this high activity is due to a benzene-benzene interaction between the pending phenyl groups within the pores and BA, leading to efficient transfer of holes. The interaction of BA with PMCR-1 was confirmed by TGA measurements (Figure S15). Furthermore, a two-phase (liquid-liquid) system if formed from water and BA. Recent reports illustrated that metal organic framework (MOF) and COF photocatalysts can achieve better H2O2 production from such binary phase system.12, 36-38, 58
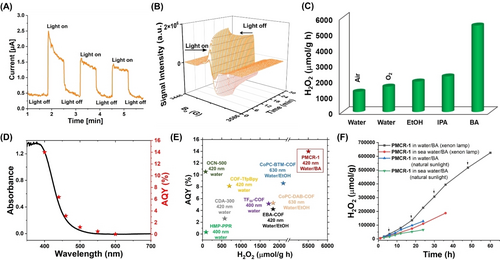
(A) Photocurrent measurement of PMCR-1; (B) EPR conduction band (CB) electron spectra of PMCR-1 in the dark and after illumination (λ>420 nm, 300 W Xe lamp); (C) Photocatalytic H2O2 production using PMCR-1 in the presence of water and different sacrificial donors (10 mg of COF in 22 mL water or 10 : 1 water:alcohol mixtures, at 25 °C, λ>420 nm, O2 purge 10 min); (D) Wavelength dependent AQY measurement in water/BA; (E) Comparison of H2O2 production and AQY with other COF and polymer based photocatalysts; (F) Long-term photocatalytic H2O2 production of PMCR-1 in water in presence of BA (10 mg of COFs in 10 : 1 water:BA (22 mL) at 25 °C and λ>420 nm, black arrow sign corresponding to O2 flow for 10 min).
The two-phase system of BA/water allows the dispersion of PMCR-1 in the organic phase, thus that H2O2 production presumably occurs at the BA/water interphase while the produced H2O2 is dissolved in the water phase (Figure S16). This avoids the back reaction by H2O2 removal, and separation from the catalyst which plays a key role for efficient H2O2 production and also inhibit H2O2 decomposition.12, 36, 37 An apparent quantum yield (AQY) of 14 % at 420 nm in water/BA was found for PMCR-1 in presence of O2 (Figure 3D). The activity of PMCR-1 in H2O2 production is favorably comparable to other reported COFs in the literature (Figure 3E and Table S2).
Motivated by this H2O2 production activity, the practical applicability of PMCR-1 was tested for long-term H2O2 production in pure and seawater in presence of BA. Production of H2O2 from the water/BA two-phase mixture steadily increased over time (226,450 to 625,790 μmol g−1 after 24 to 60 h, respectively) (Figure 3F). Also, under natural sunlight a high and steady production of H2O2 is observed reaching 129,028 μmol g−1 after 24 h. The catalyst can be easily isolated from the organic phase of the reaction mixture by filtration. After washing and drying, PXRD and XPS pattern showed almost no change in the structure of PMCR-1 after the long-term activity test (Figures S17 and S18). HRTEM images displayed the retained morphology and crystallinity after long-term photocatalysis (Figure S19). 1H NMR analysis of the filtrate confirmed the formation of benzaldehyde (Figure S20). The amount of benzaldehyde is slightly higher than the amount of H2O2 produced indicating the partial decomposition of H2O2 in the long-term production process.37, 58
To gain more insight into the photocatalytic H2O2 production mechanism, different conditions were applied during the tests. Under Ar atmosphere, the production of H2O2 was nearly not detectable suggesting that it occurs via oxygen reduction. In absence of light, no H2O2 production was observed even after 48 h proving the photocatalytic process.
Furthermore, different trapping agents such as benzoquinone (BQ) as superoxide radical (⋅O2−), t-butyl alcohol (TBA) as hydroxyl radical (⋅OH), and Ag(NO3) as electron scavenger, respectively, were used to further reveal the mechanism of the photocatalytic reaction. Addition of BQ quenched the H2O2 production, suggesting that ⋅O2− is involved in the process. H2O2 production also decreased after adding Ag(NO3), indicating that photogenerated electrons play a crucial role in the oxygen reduction reaction. On the other hand, addition of TBA has not much influence on the H2O2 production, which shows that ⋅OH radicals are not taking part in the photocatalytic process (Figure S21). To elucidate the formation of the ROS (e.g. ⋅O2− and ⋅OH), EPR measurements were performed. 5,5-dimethyl-1-pyrrolidine N-oxide (DMPO) was used as a spin trap to detect the ROS. A strong signal of ⋅O2− was distinguished, which indicates reduction of O2 upon illumination while no signal can be seen in absence of light (Figure 4A). Finally, possible O2 interaction sites and binding energies (BE) on PMCR-1 were investigated by configurational bias Monte Carlo (CBMC) simulation (Figure S22). The triazine (CH⋅⋅⋅O: 2.36–2.93 Å) and quinoline moities (CH⋅⋅⋅O: 2.68–2.94 Å), as well as the pendent phenyl group (CH⋅⋅⋅O: 2.68–2.94 Å) of PMCR-1 seem to be preferred binding sites for the O2 molecules. These results strongly suggest that photocatalytic H2O2 production is indeed occurring by reduction of O2 as initial step and produced superoxide radicals along with another electron and two protons (from alcohol to aldehyde) to generate H2O2 (Figure S23).
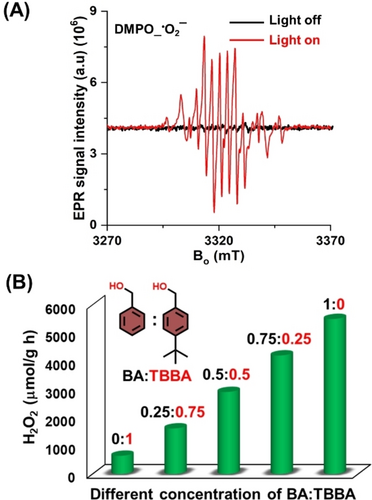
(A) EPR trapping experiment with DMPO which showed the formation of DMPO-⋅OO spin adduct (g=2.006; AN=13.2, and AH=10.1; (B) H2O2 production from sacrificial donor BA : TBBA mixtures.
To gain further insight into the interaction of the COF with the sacrificial reductant, alcohols with a different sterical hindrance were investigated. When the bulky sacrificial donor 4-t-butylbenzylalcohol (TBBA) was used, the production of H2O2 was found to be much lower (700 μmol h−1 g−1) than for pure BA (Figure 4B). After addition of different amounts of BA to TBBA (0 : 1, 0.25 : 0.75, 0.5 : 0.5, 0.75 : 0.25, 1 : 0 of BA : TBBA), the H2O2 production increased nearly linearly (from 700 to 5500 μmol h−1 g−1) (Figure 4B). This result indicates that π-π interactions of the COF with the SA might be an important factor for an efficient hole transfer, as such interactions will be much reduced when a sterically hindered alcohol is used.
Benzyl alcohol to benzaldehyde oxidation should be however rather regarded as model oxidation reaction as, even when coupled to H2O2 production, the economic feasibility of this process is questionable. However, this might change for light driven, selective oxidations of other alcohols and further chemicals. Therefore, different benzyl amines and thiols were applied to investigate the versatility of the presented photocatalytic system. Prior works54, 60 have reported that benzyl amine generates N-benzyl-1-phenylmethanimine when applied as sacrificial agent via phenylmethanimine (PhCH=NH) in photocatalytic H2O2 production (Figure S23). Here, the same reaction was attempted in the binary phase system water/benzyl amine while H2O2 was detected in the water phase. Notably, when natural sunlight was used as a light source, already with a very low amount of benzyl amine in water (0.01 : 1), a H2O2 production of 4529 μmol g−1 was observed after 1 h under O2 atmosphere. Similarly, thioanisole in water (0.1 : 1) produces a two-phase system but in this case H2O2 production is lower (905 μmol h−1 g−1) compared to BA or benzyl amine.
Inspired by these findings, the aerobic oxidation of further molecular compounds was attempted, as the photocatalytic oxidation produce valuable products for the chemical and pharmaceutical industry.59 Recently, various heterogeneous catalysts were used for such photocatalytic selective oxidations, however, stability and thus reusability are critical issues in the presence of highly reactive ROS. In this respect it can be assumed that the strong covalent bonds in PMCR-1 will also be beneficial to ensure its stability in presence of ROS. At first, oxidation of different benzylamine derivatives was tested using PMCR-1 as catalyst (Table 1). PMCR-1 was dispersed in CH3CN and the amine and purged with O2 for 10 min. For pure benzyl amine complete conversion was observed after 4 h. Under inert conditions (Ar purge) a much lower conversion was observed (12 % after 4 h, note that O2 cannot be completely removed from the reaction system). For the other amines, substrates with electron-donating groups (EDGs) show better activity compared to electron-withdrawing groups (EWGs) (Table 1). Again, sterically more demanding groups yield to lower catalytic conversions. Additionally, the oxidation of sulfides was tested using PMCR-1. Also, for this reaction a higher activity for substrates with small and EDGs groups is observed (Table 2).
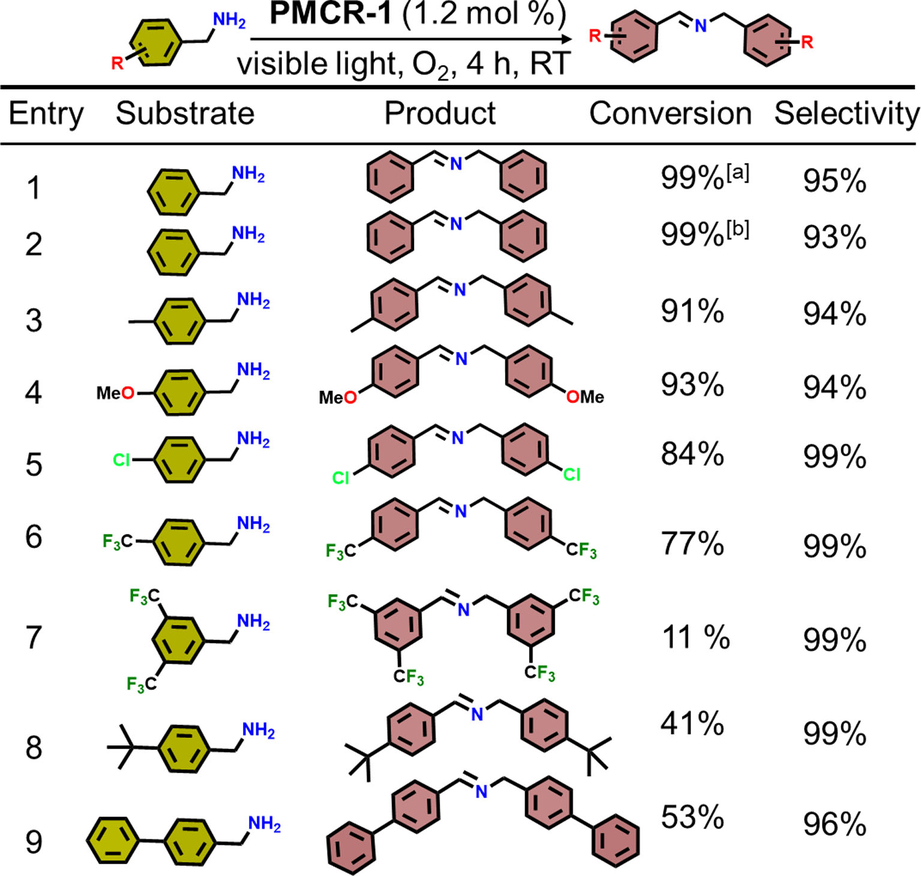
- Reaction conditions: benzylamines (0.2 mmol), PMCR-1 (5 mg), CH3CN (10 mL), visible light, RT. Conversion determined by 1H NMR spectroscopy. [a]=sunlight, [b]=visible light (λ>400 nm).
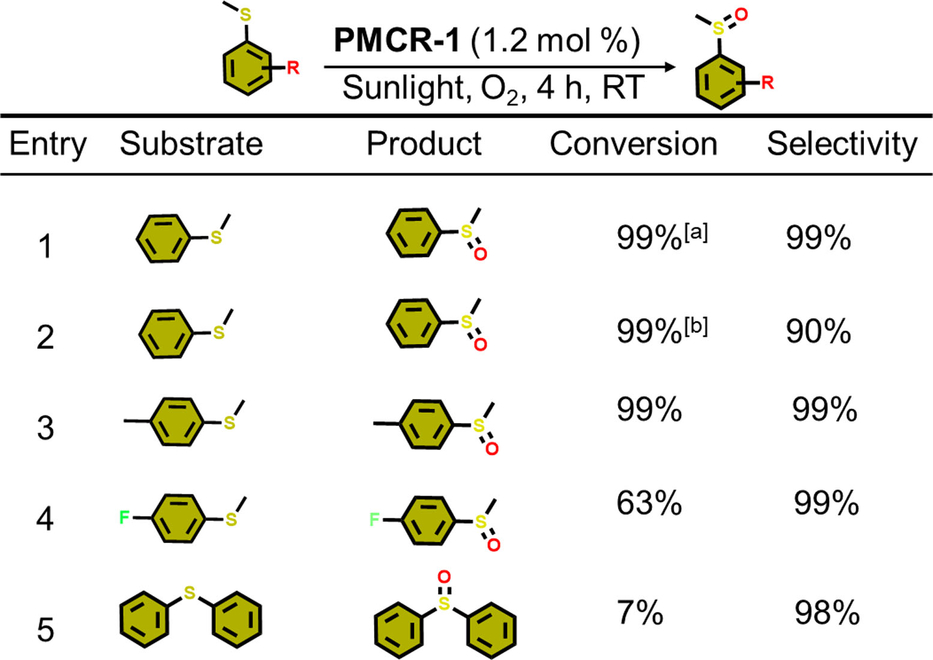
- Reaction conditions: thioanisole (0.2 mmol), PMCR-1 (5 mg), CH3CN (10 mL), sunlight, RT (temperature vary from 25–30 °C). Conversion determined by 1H NMR spectroscopy. [a]=in O2, [b]=in air.
These results are pointing to a size-selective effect as bulkier substrates oxidize much slower than smaller ones. For larger substrates like t-butylbenzylamine (Entry 8) and phenylbenzylamine (Entry 9), the conversion is comparably low (Table 1). When a larger EWG substrate e.g. 3,5-bis(trifluoromethyl)benzylamine (BTBA) was used the conversion is even lower (Entry 7, 8, Table 1). Similar results are found for sulfides: smaller and linear substrates are easily converted (>60 %), while the bulkier substrate diphenylsulfane showed just very low conversion (≈7 %) after 4 h (Table 2).
PMCR-1 has uniform microporous channels (average pore size ≈1 nm) and such size-selective conversion is reported for a 2D COF for the first time, to the best of our knowledge. Size-selective chemical conversion has been described in MOFs, [60, 61] but so far only for two 3D COFs.62, 63 To further verify this size-selective conversion, mixtures of substrates, namely benzyl amine/BTBA and thioanisole/diphenylsulfane were investigated and their conversion was monitored over time (Figure 5). After 1 h, the oxidation of benzyl amine has progressed to ≈50 % while for BTBA just 3 % conversion is found which even after 4 h has not increased over 11 % (Figure 5A). Similarly, for diphenylsulfane even after 4 h, the conversion has not increased over 9 %, whereas thioanisole shows almost full conversion. The molecular size of BTBA and diphenylsulfane are 1.2×1 nm and 1.2×1.2 nm (width x height) (including van der Wall radii), respectively, which is very close to the pore aperture of PMCR-1 thus yielding severs transport limitations inside the COF channels (Figure 5).
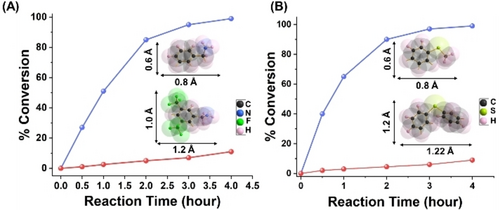
Conversion vs time plot for aerobic oxidation of small and larger substrates: (a) benzylamine vs BTBA, and (b) thioanisole vs diphenylsulfane.
Conclusion
In summary, a COF prepared by the multicomponent Povarov reaction (PMCR-1) exhibited a very high activity for photocatalytic H2O2 production as well as for size-selective photocatalytic oxidations. PMCR-1 can be used as catalyst for a range of important aerobic oxidation reactions including alcohols to aldehydes, amines to imines and sulfides to sulfoxides. High activities are observed for substrates considerably smaller than the pore aperture of PMCR-1, while bulkier substrates show much lower conversion. These findings provide insight into the utilization of surface area and pore volume of COFs in photocatalytic processes.
Acknowledgments
Support from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy—EXC 2008-390540038—UniSysCat and the BMBF (Fördermaßnahme CO2-WIN, Förderkennzeichen 033RC024, PRODIGY) is acknowledged. P.D. thanks the Alexander von Humboldt foundation for a postdoctoral fellowship. DFG funding is acknowledged for HRTEM measurement at TU Berlin. Thanks to Dr. Johannes Schmidt, Dr. Sarah Vogl, Christina Eichenauer and Maria Unterweger for their assistance. The author thanks to Prof. Jabor Rabeah for EPR measurement and helpful suggestions. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.