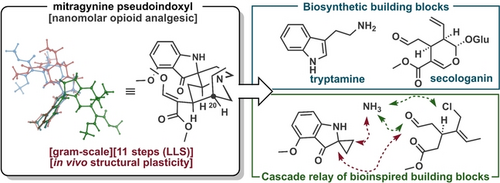
Total Synthesis and Structural Plasticity of Kratom Pseudoindoxyl Metabolites**
A previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv-2023-62vzz-v2).
Graphical Abstract
Although opioids have been used in the treatment of pain for centuries, they cause various adverse effects and addiction. Recently, kratom alkaloids emerged as promising analgesic alternatives for pain management with considerably fewer side effects. The first scalable total synthesis of mitragynine pseudoindoxyl, the most potent atypical opioid kratom metabolite, is now reported, as well as the discovery of its structural plasticity.
Abstract
Mitragynine pseudoindoxyl, a kratom metabolite, has attracted increasing attention due to its favorable side effect profile as compared to conventional opioids. Herein, we describe the first enantioselective and scalable total synthesis of this natural product and its epimeric congener, speciogynine pseudoindoxyl. The characteristic spiro-5-5-6-tricyclic system of these alkaloids was formed through a protecting-group-free cascade relay process in which oxidized tryptamine and secologanin analogues were used. Furthermore, we discovered that mitragynine pseudoindoxyl acts not as a single molecular entity but as a dynamic ensemble of stereoisomers in protic environments; thus, it exhibits structural plasticity in biological systems. Accordingly, these synthetic, structural, and biological studies provide a basis for the planned design of mitragynine pseudoindoxyl analogues, which can guide the development of next-generation analgesics.
Introduction
Pain management is one of the oldest challenges for medicine, and opioids targeting μ-opioid receptors (MORs) play a fundamental role in the treatment of moderate-to-severe acute and chronic pain. Although opioid agonists are still the most effective analgesics, there is a growing concern about the safety of their long-term administration owing to various adverse effects, such as respiratory depression, constipation, and addiction. Additionally, the current upsurge in opioid abuse, coupled with the spread of illicit synthetic opioids, has reached an epidemic level in the United States,1 wreaking havoc on public health, society, and the economy. In addition to introducing new legislation and public education, the development of safer analgesics is a major goal in pain management.2
In this context, alkaloids from Mitragyna speciosa (commonly known as kratom) have recently emerged as a promising analgesic alternative for pain management with considerably fewer side effects than those associated with opioids.3 Kratom is a tropical tree-like herb native to Southeast Asia that has been used for centuries for its distinctive psychotropic properties.4 Kratom has been noted to have dose-dependent stimulant and opioid-like effects, and its illicit use to treat chronic pain and drug dependence is on the rise globally (>15 million estimated kratom users in the US alone).5 The predominant natural products in kratom are indole alkaloids, mainly corynanthe-type monoterpenoids. While these mitragyna alkaloids are structurally significantly different from the morphine skeleton, they do display antinociceptive effects, which are supported by their ability to bind to opioid (i.e., MORs, DORs, KORs, NORs)6 and other receptors (e.g., serotonin receptors).7 Systematic investigations have also shown that the opioid agonistic effect of Mitragyna speciosa cannot be fully explained by that of its main alkaloid, mitragynine (1; 230 nM for MOR; Figure 1). Finally, more potent analgesics, which were the oxidative metabolites of mitragynine, were identified in in vitro assays. Accordingly, the main alkaloid metabolizes in vivo8-10 to 7-OH-mitragynine (2; 37 nM for MOR), which is followed by a subsequent 1,2-semipinacol rearrangement in human plasma to the more potent mitragynine pseudoindoxyl (3; 0.8 nM for MOR, G-protein-biased MOR partial agonist). Nevertheless, hindering the completeness of the oxidative metabolic picture of mitragynine (1), another metabolite in addition to mitragynine pseudoindoxyl (3) was also detected in the semipinacol rearrangement step, but its structure and possible opioid effect have not been determined to date owing to its low occurrence in human plasma.9
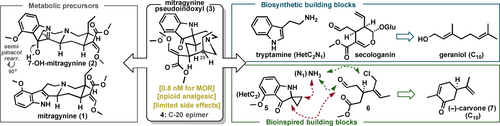
Introduction and comparison of distinct pathways toward pseudoindoxyl metabolites of kratom alkaloids.
Most importantly, however, animal studies have shown that mitragynine pseudoindoxyl (3) may avoid some of the key problems of current opioid therapies, as antinociceptive tolerance, physical dependence, and respiratory depression were not observed.11 To gain insight into the underlying molecular mechanism of its distinct signaling profile, high-resolution cryo-electron microscopy (cryo-EM) structural studies on the MOR-Gi1 complex with 3 and molecular dynamics simulations have been performed.12 Furthermore, combined double electron–electron resonance (DEER) and single-molecule fluorescence resonance energy transfer (smFRET) studies were recently conducted to investigate the molecular underpinnings of MOR activation and MOR mediated G protein signaling. Of interest, when bound to 3, there was no shift toward active conformations of MOR, which affected its downstream signaling efficacy and selectivity.13
Although the syntheses of kratom's major indole alkaloids4, 14 and their oxindole derivatives15 have already received considerable attention from the synthetic community, total syntheses of mitragynine pseudoindoxyl derivative 3 and kratom's other minor alkaloids (e.g., the recently reported speciogynine derivative 4)16, 17 have not yet been revealed. Nevertheless, Sorensen and co-workers could synthesize the methoxy analogue of 3 by the ingenious construction of the challenging spiro-fused pseudoindoxyl system.18 However, their interrupted approach based on the Ugi reaction was limited to highly electron rich aromatic derivatives and did not allow the synthesis of the natural product 3 itself. Thus far, access to mitragynine's oxidative metabolites 2 and 3 has relied exclusively on low-yielding biomimetic semisynthesis from mitragynine (1), which generates the pseudoindoxyl ring in a 1,2-semipinacol rearrangement.11, 19 Although these and related studies demonstrated the power of late-stage functionalization strategies (e.g., selective oxidation and site-selective aromatic C−H functionalization),20, 21 the attainable chemical space did not allow extensive structure–activity relationship (SAR) studies or deep-seated modifications of this unique pseudoindoxyl scaffold, thus hampering further advances in drug development.22, 23
Given the enormous therapeutic potential and the lack of an enabling synthetic platform for SAR studies, we aimed to develop a scalable24 and modular route to pseudoindoxyl opioid 3. Of all the possible disconnections of its spiro-tetracyclic backbone, the one that maximizes convergence and skeletal simplification and whose forward synthesis is concise and potentially scalable was chosen. Figure 1 illustrates the process through which we disconnected mitragynine pseudoindoxyl (3) into two subunits, a dubbed “oxidized tryptamine” fragment 5 and a secologanin analogue25 6 that was anticipated to arise from (−)-carvone (7).26 A cascade process was then devised that would i) strictly avoid the use of protecting groups and harness the natural reactivities of the two key building blocks and ii) forge the CD ring system and the critical stereogenic spirocyclic center. Furthermore, our strategy was based on a stereocenter derived from (−)-carvone (7), of which its stereochemical information could be propagated to diastereoselectively adjust two other stereocenters. The decision to implement this diastereoselective cascade relay was also underpinned by the computational analysis of the possible diastereomers of 3 (see the Supporting Information for details). It turned out that the thermodynamically most stable stereostructure was that of 3, which was also the product of the biosynthetic pathway.27 Finally, we believed that our secologanin analogue could further broaden the synthetic landscape, as it could provide access to both diastereomeric kratom pseudoindoxyls of the corynanthe type, allo (mitragynine) and normal (speciogynine) isomers, by selective hydrogenation of its olefinic bond.28, 29 As such, this synthetic development was inspired by the manner in which nature assembles mitragynine pseudoindoxyl (3), including rapid complexity generation through cascade reactions of tryptamine and secologanin analogues, step and redox economy, divergency and protecting-group-free assembly.30 Herein, we report the first total synthesis of mitragynine pseudoindoxyl (3) and its diastereomer, speciogynine pseudoindoxyl (4).
Results and Discussion
Our investigation began with the construction of the chiral secologanin-like building block 6 using (−)-carvone (7) as a chiral terpenoid starting material. To this end, we developed an operationally simple and scalable synthetic route that features a relay of chemoselective transformations (Figure 2).31 First, the homologation of (−)-carvone (7) was achieved in a two-step procedure: Ene-type chlorination32 of the electron-rich double bond with trichloroisocyanuric acid (TCICA) was followed by a copper-catalyzed C−C bond-forming step to form homocarvone (8). Notably, the latter step was selective, as no 1,2- or 1,4-addition products of enone were observed under optimized conditions. Then, two orthogonal oxidation reactions were carried out; a nucleophilic epoxidation selectively transformed the electron-deficient double bond into an epoxide, and a second ene-type chlorination formed allylic chlorine 9 with high Z selectivity, presumably due to syn-pentane strain.33 After obtaining the desired intermediate 9 in an operationally simple way in a useful yield (39 % yield over 4 steps, avg. 79 %), two C−C bond cleavages remained to successfully remodel the initial terpene skeleton34 and achieve the open-chain C9 carbon core of the envisioned secologanin building block 6. To prepare for these C−C bond-breaking reactions, mild acidic hydrolysis of the epoxide moiety was carried out at room temperature, which retained the sensitive allylic chlorine moiety. Then, the resulting mixture of diastereomeric ketone diols was exposed to an excess of sodium periodate to effect the required C−C cleavages and provide the open-chain carboxylic acid 10. The handling of this multifunctional and reactive molecule 10, however, turned out to be challenging owing to the presence of electrophilic and nucleophilic sites combined with its apparent ability to form cyclic acetals or esters. After careful experimentation, simple ester formation with diazomethane was found to address this problem, as it promoted clean methylation to complete the sequence without the need for further purifications (71 % yield over 3 steps, avg. 89 %). In summary, a scalable, protecting-group-free route to secologanin analogue 6 was achieved from (−)-carvone (7) in only seven steps. Each of the reaction steps was performed on a decagram scale, and only one purification step was involved, attesting to the robustness and potential further scalability of the sequence.
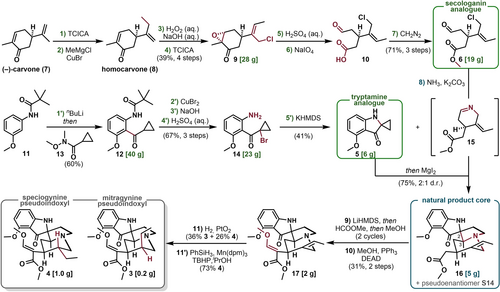
Bioinspired synthesis to form pseudoindoxyl metabolites on a gram scale. Reagents and conditions: 1) TCICA (0.33 eq.), CHCl3, 5 to 25 °C; 2) CuBr (0.25 eq.), MeMgCl (1.0 eq.), THF, −65 °C; 3) H2O2 (aq.) (1.3 eq.), NaOH (aq.) (0.27 eq.), MeOH, −10 to 0 °C; 4) TCICA (0.24 eq.), CHCl3, 5 to 23 °C; 5) 50 % H2SO4 (aq.), THF, 5 to 23 °C; 6) NaIO4 (3.0 eq.), THF/H2O, 5 to 23 °C; 7) CH2N2, Et2O, 5 to 10 °C; 1′) nBuLi (2.2 eq.) then 13 (1.0 eq.), THF, 5 to 25 °C; 2′) CuBr2 (2.2 eq.), EtOAc, reflux; 3′) NaOH (2.0 eq.), MeOH, 25 °C; 4′) 25 % H2SO4 (aq.), MeOH, 65 °C; 5′) KHMDS (2.0 eq.), THF, 5 to 23 °C; 8) 6 (2.2 eq.), NH3 (1 atm), K2CO3 (exc.), CaCl2 (exc.), CH2Cl2, 23 °C, then the addition of 5 (1 eq.) and MgI2 (1 eq.) in THF, 23 °C; 9) LiHMDS (5.0 eq.), then HCOOMe (10 eq.), THF, −40 °C, then the addition of MeOH, 23 °C (2 cycles); 10) MeOH (2.0 eq.), DEAD (1.0 eq.), PPh3 (1.0 eq.), THF, 23 °C; 11) H2 (1 atm), PtO2 ⋅ H2O (50 m/m%), NaHCO3 (exc.), TFE, 23 °C; 11′) PhSiH3 (3.0 eq.), Mn(dpm)3 (0.25 eq.), TBHP in decane (1.0 eq.), iPrOH, 23 °C. TCICA=trichloroisocyanuric acid, KHMDS=potassium bis(trimethylsilyl)amide, exc.=excess, LiHMDS=lithium bis(trimethylsilyl)amide, DEAD=diethyl azodicarboxylate, TFE=2,2,2-trifluoroethanol, Mn(dpm)3=tris(2,2,6,6-tetramethyl-3,5-heptanedionato)manganese(III), TBHP=tert-butyl hydroperoxide.
Next, we focused on synthesizing the spirocyclic cyclopropane–indoxyl building block 5. Initial attempts to accomplish the synthesis of 5 on a gram scale were unsuccessful owing to the high reactivity and sensitivity of this compound (see the Supporting Information for details); at best, only milligram amounts of spiro-pseudoindoxyl derivative 5 were obtained. Upon extensive experimentation, we found an alternative synthetic tactic using mild enough conditions to provide 5 in sufficient quantities. As shown in Figure 2, commercially available aniline 11 was used to construct the reactive spirocyclic building block 5 in five steps. The synergistic effect35 of the meta-positioned directing groups of 11 in directed ortho-metalation resulted in the construction of the contiguously functionalized 1,2,3-aromatic system 12 after acylation of the lithiated species with 13, in 60 % yield on a decagram scale. Next, 12 was converted into anilino-α-bromo compound 14 through a dibromination/base-induced ring closure/acidic hydrolyzation sequence in 67 % yield, which set the stage for the last spiro-pseudoindoxyl formation step. After screening a variety of different reagents (see the Supporting Information for details), KHMDS was found to promote the formation of the desired tryptamine analogue 5 with good overall efficiency on a gram scale.
With ample quantities of the two building blocks 5 and 6, we began to explore the key cascade relay sequence needed to construct the spirocyclic tetracyclic core found in mitragynine pseudoindoxyl (3). As both bioinspired analogues were endowed with reactive functionalities, it seemed less apparent that they could be woven efficiently and selectively together without using protecting groups. Nevertheless, we reasoned that the functional-group incompatibility could be addressed by developing a cascade relay sequence and leveraging a heterocyclic intermediate derived from secologanin analogue 6 in a cascade process. This fleeting cyclic imino compound 15 was considered to be a suitable substrate for the ring-opening and Mannich reaction cascade with 5 to produce the spirocyclic tetracyclic core of 3.
However, this cascade-relay strategy was dependent on the feasibility of producing the unstable cyclic imine intermediate 15. Although the synthesis and isolation of 15 were difficult owing to the formation of oligomeric and polymeric side products upon synthesis and storage, we were able to find appropriate basic and anhydrous conditions to form 15 with consistently high conversion and selectivity on a multigram scale (see the Supporting Information for details). Then, without an aqueous workup or removal of solvent, this unstable cyclic imine 15 was carried onwards for the ensuing cascade reaction with the cyclopropyl building block 5. Gratifyingly, the formal [3+2] cycloaddition step36 afforded the desired spirocyclic 5-5 pseudoindoxyl ring system at room temperature. Thus, there was no need to apply forcing reaction conditions reported by Pierce (i.e., 200 °C)36 for the analogous intramolecular reaction or by Carreira37 (i.e., 80 °C) for the conversion of less reactive oxindoles analogues. Accordingly, milder reaction conditions alleviated the problem of decomposition or isomerization38 of 15 in our synthetic approach. Of the possible four diastereomers, the desired isomer 16 was obtained in 50 % yield, with its pseudoenantiomer S14 (see the Supporting Information for the elucidated structure) in 25 % yield as an inseparable mixture (5 g scale). Although the installation of the two stereocenters was not fully diastereoselective, we anticipated that the forward reaction conditions would concomitantly epimerize those C2 and C3 stereocenters to the desired configurations. At least two important features of this cascade relay were noteworthy: i) Our imine formation/alkylation tactic enabled the facile synthesis of cyclic imines, which usually requires extensive redox and protecting-group manipulations;39 and ii) the overall cascade sequence enabled rapid complexity generation and good convergence, as four bonds, two rings and two stereocenters were formed from two comparatively complex building blocks.40
With the pentacyclic core of mitragynine pseudoindoxyl (3) in hand, two seemingly straightforward tasks remained: the installation of the methyl enol ether moiety onto the acetyl side chain and stereoselective reduction of the alkene. To this end, an α-formylation protocol was adapted to our substrate; this protocol does not require preventive but only transient protection of the pseudoindoxyl NH. Accordingly, the double deprotonation of 16 with excess LiHMDS was followed by the addition of methyl formate and quenching with methanol; this produced the α-formylated product as a mixture of isomers (see the Supporting Information for details) and ensured the deprotection of the transient N-formyl group. Then, the selective methylation of the enol tautomer was explored using commonly used alkylation protocols (see the Supporting Information for details). However, these methods were plagued by poor and inconsistent yields owing to N-methylated side-product formation. To circumvent this problem, we used the Mitsunobu reaction, which allowed chemoselective O-alkylation and afforded 17 in good yield and quantity (gram scale). Finally, the stereoselective reduction of 17 was addressed to complete the total synthesis of the targeted mitragynine metabolite 3, alongside its recently reported diastereomer, speciogynine pseudoindoxyl (4).
Many conditions and catalysts were screened to maximize the diastereoselectivity of the reduction process in both possible directions. We found that use of the Adam catalyst was necessary to provide our primary target, the allo-type mitragynine pseudoindoxyl (3), with modest diastereoselectivity. To accomplish the selective reduction of 17 to diastereoisomer 4, a radical stepwise reduction was chosen to provide the thermodynamically more stable diastereoisomeric product. The HAT-type reduction, pioneered by Shenvi,41 afforded the “normal-type” speciogynine pseudoindoxyl (4) in 73 % yield in a diastereoselective manner.
Although our primary goal was to access the kratom atypical opioid 3 in a scalable manner, this endeavor also provided new insight into its molecular properties. During the optimization of the last reduction step (see the Supporting Information for details), we noticed that this natural metabolite was labile under various reaction and isolation conditions, which was also manifested in diminished yields. Prompted by these observations and having a useful quantity of 17, we aimed to thoroughly study the reaction mixture of the last step and isolate minor or even trace components. These efforts ultimately resulted in the identification of two unknown diastereomers of mitragynine pseudoindoxyl (18 in 5 % yield and 19 in 4 % yield), which most likely arose from a retro-Mannich/Mannich-type rearrangement of the spiro-pseudoindoxyl moiety (Figure 3A). We then identified conditions and methods that allowed the isolation and structural elucidation of those stereoisomers. The NMR structure elucidation of these isomers showed that they are spirocyclic diastereomers with allo and epiallo configurations; thus, the latter stereoisomer is the not-yet-known spiroindoxyl derivative of speciociliatine. Next, it was found that aprotic solvents impeded and polar protic solvents facilitated the isomerization and shifted the equilibrium (e.g., 3 : 18 : 19≈6 : 1 : 3 in HFIP, 23 °C). Most importantly, we found that the thermodynamic equilibrium of the three stereoisomers (3 : 18 : 19≈7 :2 : 1) could be reached within 20 min at 37 °C in Krebs–Ringer solution, which mimics physiological conditions (Figure 3B). Additional insight was obtained from the computational assessment of the relative free energies of the (protonated) isomers. First, it confirmed the experimental relative thermodynamic stabilities; second, it explained the observed experimental difference between protic and aprotic media, that is, protonation of the tertiary amine was found to reduce the energy difference between the isomers (see the Supporting Information for details).
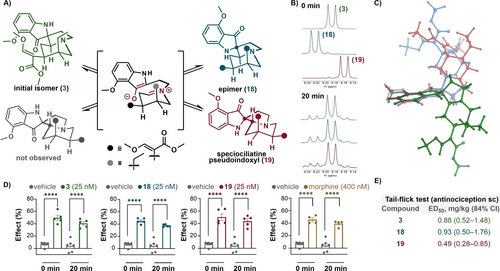
Isomerization of mitragynine pseudoindoxyl (3) and related studies. A) Retro-Mannich/Mannich isomerization and structure of the isomers obtained. B) NMR spectra: Single isomers 3, 18 and 19 were introduced into physiological-like media, and their isomerization was monitored with 1H NMR measurements, as illustrated by the shifts of characteristic aromatic signals (starting materials, 0 min (up) vs. 37 °C, pH=7.4, 20 min (down)). C) Superimposed structures of the calculated lowest energy conformations of 3 (green), 18 (blue), and 19 (red). D) Results of the in vitro mouse vas deferens bioassay comparing the effect of single isomers: Test or reference compounds were added to the MVD immediately or 20 min after solution preparation. The effect on MVD smooth muscle contractions was assessed after 10 min. Statistical significance was accepted based on one-way ANOVA with the Tukey multiple comparison test, p<0.05 (**** p<0.0001). Data are presented as the mean±S.E.M. of 5 or 6 tissues. E) Results of the in vivo radiant heat tail flick assay in mice comparing the effect of single isomers: The antinociceptive effect of test compounds was assessed 20, 30, 60 and 120 min after s.c. administration at various doses (see the Supporting Information for details). The antinociceptive potencies (ED50) were calculated from linear dose response curves. The ED50 values and 84 % confidence intervals were calculated at the most effective time point, namely, 20 min after s.c. administration.
While analogous and kinetically more stable spiro-oxindoles are known to exist in at least two isomeric forms,42 only one structural isomer has been observed for spiro-pseudoindoxyl systems to date.43 Although Takayama et al. hypothesized the formation of four possible stereoisomers 30 years ago in their pioneering work, only one pseudoindoxyl diastereomer was isolated after the 1,2-semipinacol rearrangement of the biogenetic precursor 7-OH-mitragynine (2).19
A critical question that remained at this stage concerned the opioid activity of these stereoisomers. Our computational studies of the stereoisomers suggested that the shapes of these molecules are significantly different (Figure 3C); thus, a profound difference in their biological activities might be expected. Nevertheless, on the basis of the observation of the rapid stereoisomerization phenomenon, we anticipated that all stereoisomers might show the same activity in biological systems. Thus, we initiated comparative in vitro and in vivo studies for each single stereoisomer. First, we evaluated the opioid-receptor-mediated effects of these isomers (3, 18, and 19) in the mouse vas deferens (MVD) assay44, 45 (Figure 3D). All compounds showed naloxone-sensitive inhibitory effects on MVD muscle contractions, with significantly higher potency than morphine. Furthermore, Student's t-test showed no difference in the inhibitory effect between stereoisomers administered immediately after solution preparation or 20 min later. Accordingly, this study suggests that the opioid effect of those isomers is related to their dynamic stereochemical equilibrium. Next, we examined the acute analgesic effect of these isomers. Thus, a thermal pain model, the radiant-heat tail-flick assay,11, 46 was selected and performed in the same mouse strain used for MVD experiments (Figure 3E). Every experiment comprised five series of measurements, one before and four after subcutaneous drug administration, carried out at 20, 30, 60 and 120 min intervals. The antinociceptive effect was assessed 20 min after s.c. drug administration since the antinociception of the compounds peaked at that time (see Supporting Information , Figure S35). Importantly, there was no significant difference between the ED50 values of the stereoisomers, as indicated by the overlap in 84 % confidence intervals. Accordingly, we found that mitragynine pseudoindoxyl (3) actually exists and acts as a dynamic ensemble of stereoisomers in the protic environment and, what is perhaps even more important, in biological systems. This finding has many important implications that are vital to the interpretation of previous results and can shape future drug developments: i) The elusive, unidentified 7-OH-mitragynine metabolite in human plasma described by McCurdy9 is likely to be the above identified stereoisomer(s) of 3; ii) mitragynine pseudoindoxyl (3) displays structural plasticity, and the evolving molecular ensemble could target additional receptors. As shown by biological and pharmaceutical investigations47 for other ligands, an isomerization-induced conformational and structural heterogeneity may influence the ligand's affinity and efficacy to target receptors and affect its ADMET properties; iii) the structural plasticity of 3 might be important, or even critical for its low efficacy, G-protein-biased agonist activity. Additionally, assuming that the time window of the isomerization of 3 is comparable or smaller than that of the conformational change of MOR, the isomerization should be taken into consideration for molecular docking48 and future drug development; iv) the local biological environment (e.g., pH, metal ions) can alter the potency of the pseudoindoxyl ensemble due to the shift of the equilibrium of stereoisomers; v) and the oxidative metabolism of speciociliatine to its pseudoindoxyl derivative (the C3 epimer of mitragynine)49 is predicted to result in the same stereomeric mixture as described herein, which also implies an unusual convergent metabolic pathway for kratom alkaloids.
Conclusion
In summary, the first scalable and chiral-pool-based synthetic routes to members of kratom pseudoindoxyls have been developed by using oxidized tryptamine and secologanin analogues. The highly convergent strategy, the deliberate minimization of the use of protecting groups, and late-stage divergency allowed the rapid construction of targeted natural products 3 and 4 on a gram scale. While our synthetic route mainly benefited from effective strategy and tactics, several methodological advances were also achieved: i) The chemoselective homologation of carvone and a stereoselective ene-type chlorination formed the basis for a streamlined synthesis of our key secologanin building block; ii) a complexity-generating imine formation/alkylation/intermolecular [3+2] cascade relay was developed, which served as a cornerstone of our strategy; iii) Mitsunobu-type alkylation and HAT functionalization of late-stage pseudoindoxyl intermediates enabled the preparation of the natural products and illuminated the possibility of preparing diverse unnatural analogues not attainable by semisynthesis. As an unexpected spin-off of the synthetic efforts, we discovered that mitragynine pseudoindoxyl (3) exists and acts not as a single molecular entity but as a dynamic ensemble of stereoisomers in protic environments and, what is perhaps even more important, in biological systems. Accordingly, these synthetic, structural, and biological studies provide a basis for future functional modifications of mitragynine pseudoindoxyl (3), which can guide the development of next-generation analgesics.
Acknowledgments
Financial support provided by grant NKFIH FK 138300 is gratefully acknowledged. Project no. 1015948 has been implemented with the support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development and Innovation Fund, financed under the KDP-2020 funding Scheme (P. A.). This study was also supported by the ÚNKP-21-2 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund (B. B. M.). We thank Ádám Levente Póti for productive discussions, András Miklós Kotschy for proofreading experimental procedures, and the Servier Research Institute of Medicinal Chemistry and Gitta Schlosser for the HRMS measurements.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.