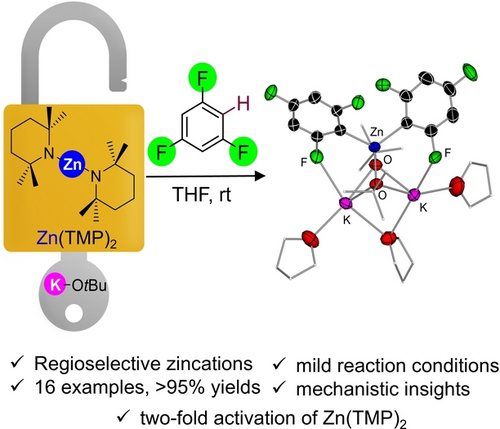
Alkali-Metal-Alkoxide Powered Zincation of Fluoroarenes Employing Zinc Bis-Amide Zn(TMP)2
Graphical Abstract
Unlocking the metalation potential of Zn(TMP)2 the addition of KOtBu enables the activation of its amide groups to promote the regioselective zincation of a range of fluoroarenes forming higher order potassium zincates. This methodology can even be extended to non-activated arenes toluene and benzene under mild reaction conditions.
Abstract
Reminiscent of Lochmann-Schlosser superbase recipes, the addition of two molar equivalents of KOtBu to Zn(TMP)2 (TMP=2,2,6,6-tetramethylpiperidide) transforms this mild zinc bis-amide base to a powerful metalating agent able to perform facile regioselective zincation of a wide range of sensitive fluoroarenes. Structural authentication of the intermediates post Zn−H exchange demonstrates activation of both TMP groups to form a range of higher order bis-aryl potassium zincates, isolable as solids and further functionalized in electrophilic interception reactions. Studies assessing the role of KOtBu reveal that the first equivalent undergoes co-complexation with Zn(TMP)2, enabling kinetic activation of the amide groups; whereas the second equivalent stabilizes the metalated intermediate preventing ligand redistribution. Showcasing its metalating power, this bimetallic KOtBu/Zn(TMP)2 partnership, can effect zincation of toluene and benzene at room temperature.
Introduction
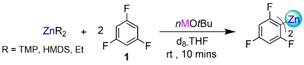
The Lochmann-Schlosser superbase or “LICKOR” reagent, typifies the ability of group 1 metal alkoxides to induce superbasicity in s-block organometallic reagents.1, 2 Challenging C−H bonds which alkyllithium reagents cannot break can often be broken by such a binary nBuLi/KOtBu mixture, since it exhibits contrasting reactivities to the single metal reagent, most importantly more powerful basicity.3-6 O'Shea has recently developed a variation of this reagent by introducing the amide TMP (2,2,6,6-tetramethylpiperidide) as a third component (referred to as the LiNK reagent).7 While the exact constitution of this base remains unknown, in solution LiTMP is formed. Remarkably, while showing enhanced reactivity, the LiNK base offers alternative and complementary selectivities to those previously found using the LICKOR superbase.7 Broadening the scope of these bimetallic approaches, Knochel has recently shown that adding variable amounts of lithium alkoxides to magnesium or zinc alkyls activates them towards Mg and Zn halogen exchange respectively in reactions with a wide range of functionalized bromoarenes (Figure 1b).8-10 Assessing the constitution of these mixtures by combining in depth spectroscopic studies with structural elucidation of key reaction intermediates prior to electrophilic interception, we have demonstrated that these processes occur by generating highly reactive lithium magnesiates and lithium zincates. Enabling the kinetic activation of the M−C bonds (M=Mg, Zn) in these compounds via this bimetallic cooperation, switches on unique reactivities unavailable to their monometallic counterparts.8, 11 These studies also revealed that several lithium ate complexes can participate in these processes, not only with different ligand sets but also in distinct Li : M ratios.11 By systematically investigating the role of alkali-metal, we have also shown the remarkable ability of alkali metal alkoxides to switch on/off complex bimetallic equilibria that profoundly affect the overall constitution of the organometallic intermediates involved in these processes.11, 12
Examples of alkoxide-mediated activation of main group organometallics for deprotonative metalation and metal-halogen exchange reactions of organic substrates.
Here we report that the zinc bis-amide, Zn(TMP)2, can also have its reactivity enhanced by the activating effect of alkali-metal alkoxides in a unique example of alkali-metal-mediation13 (Figure 1c). Finding numerous applications as a mild metalating agent for the deprotonation of relatively acidic organic substrates such as ketones and esters,14-17 this amide is available commercially in toluene solutions, though to date Zn(TMP)2 has shown little promise for zincation of less activated substrates such as arenes.18 Here, we reveal its true potential by reporting its first applications for regioselective zincation of fluoroarenes. These substrates were chosen for their synthetic relevance, as they are present in a multitude of biologically active and pharmaceutical compounds,19-21 but also for their sensitivity to highly polar organometallic reagents.3, 22 Thus, their metalation with organolithium reagents requires the use of extreme cryogenic conditions in order to avoid unwanted side reactions (e.g., benzyne formation, auto-metalation) which are favored due to the fragility of the metalated intermediates.3, 22, 23
Results and Discussion
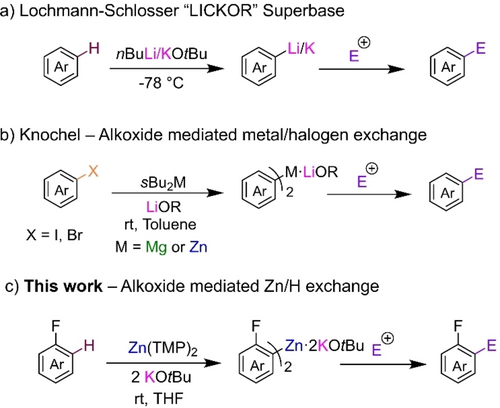
We started our investigation using 1,3,5-trifluorobenzene 1 as a model substrate (calculated pKa=31.5),24 finding that Zn(TMP)2 in THF is completely inert towards 1, even under refluxing conditions (entry 1, Table 1). Addition of one equivalent of LiOtBu led to a noticeable increase in reactivity to a 42 % conversion (entry 2) of a zincated species in just 10 minutes at room temperature. A revealing alkali metal effect was noted when moving to the heavier alkali metal congeners with addition of one equivalent of NaOtBu giving a 69 % conversion of 1 (entry 3). Most remarkably, the addition of KOtBu to Zn(TMP)2 led to the full zincation of two equivalents of 1 at room temperature in just 10 minutes (entry 4) with concomitant formation of two equivalents of TMP(H) proving both TMP groups are active towards metalation. 1H NMR monitoring of this reaction indicated that while quantitative metalation of 1 is seen, a mixture of at least four species containing Zn-C6H2F3 fragments is present in solution (see Supporting Information for details). Attempts to unequivocally ascertain the constitution of the different species present in this mixture were infructuous (see below). Related to these findings, it should be noted that our previous research probing the constitution of sBu2Mg in the presence of equimolar of lithium alkoxides has also established the presence of complex equilibria, with different bimetallic complexes coexisting in solution.11
|
||
Entry |
Base |
Conversion[a] [%] |
|---|---|---|
1 |
Zn(TMP)2 |
0[b] |
2 |
Zn(TMP)2+LiOtBu |
42[c] |
3 |
Zn(TMP)2+NaOtBu |
69[c] |
4 |
Zn(TMP)2+KOtBu |
99[c] |
5 |
Zn(TMP)2+2KOtBu |
99[d] |
6 |
TMPZnCl⋅LiCl |
13[e] |
7 |
Zn(HMDS)2+2KOtBu |
46 |
8 |
ZnEt2+2KOtBu |
43 |
- [a] Conversions determined by 1H NMR monitoring using hexamethylbenzene as internal standard [b] Reaction refluxed for 2 h [c] Multiple metalated species observed in solution [d] Single metalated species 2 a observed in solution [e] Conversion determined after 1 h at room temperature.
Interestingly, on using 2 equivalents of KOtBu, full conversion of 1 was also achieved (entry 5), but on this occasion a single organometallic complex [(THF)3K2Zn(Ar)2(OtBu)2] (2 a) (Ar=C6H2F3) was observed (see below). In order to avoid the presence of different organometallic species in solution, we opted for the use of Zn(TMP)2 with two equivalents of KOtBu as the optimal bimetallic combination for this study. We also rationalized that forming higher-order (so called because of its 2 : 1K : Zn ratio) zincate 2 a could be beneficial for forward reactivity with electrophiles.25, 26 Assessing solvent effects, full conversion is also achieved using d8-toluene although on this occasion the metalation product precipitates as a white solid, therefore THF was chosen as the preferred solvent (consult Supporting Information). No decrease in conversion of 1 is observed when using a 0.5 M solution of Zn(TMP)2 in toluene (consult Supporting Information). Using Knochel's Turbo-zinc reagent TMPZnCl⋅LiCl which has previously been employed for arene metalations, afforded only a 13 % conversion of 1 in 1 h at room temperature (entry 6).27 Lastly, replacing Zn(TMP)2 with Zn(HMDS)2 [HMDS=N(SiMe3)2, entry 7] or ZnEt2 (entry 8) gave significantly lower conversions of 1 (46 and 43 % respectively). Rapid zincation of 1 with this bimetallic combination under extremely mild reaction conditions contrasts with previous studies on the zincation of fluoroarenes that require the use of sterically demanding ligands, higher temperatures, longer reaction times and in some cases the use of Pd catalysis.19 For example, using a TMP-based potassium zincate supported by a bulky bis(amide) ligand Ph2Si(NAr*)2 (Ar*=2,6-diisopropylpheny), we reported zincation of 1 required 3 h at refluxing temperatures in THF or three days at room temperature.28 The efficient metalation of 1 under mild reaction conditions aligns better with the reactivities previously described using [NaM(HMDS)3] (M=Fe, Co) for the ferration (or cobaltation) of 1. Mechanistic studies for these reactions have revealed that initial Na−H exchange takes place followed by trapping of the trisfluorophenyl fragment by the divalent transition metal.29-32
Intrigued by these findings, we next looked at the constitution of the metalation product and the active base in this reaction in order to gain a better understanding of the potassium alkoxide role in activating Zn(TMP)2 towards deprotonation reactions.
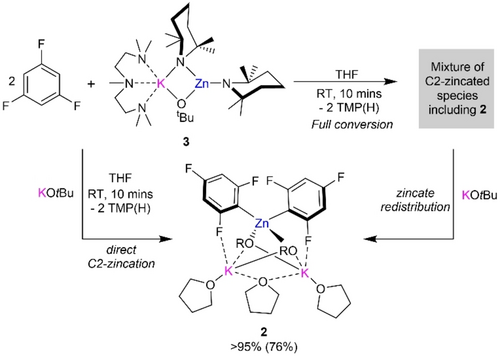
1H NMR monitoring showed that reaction of a 2 : 1 mixture of KOtBu and Zn(TMP)2 with 2 equivalents of 1,3,5-trifluorobenzene yielded 2 a quantitatively (Figure 2a) and concomitant formation of TMP(H). X-ray crystallographic studies established the bimetallic higher order (2 : 1K : Zn ratio) constitution of 2 a.25, 33 Adopting a bent K1⋅⋅⋅Zn1⋅⋅⋅K2 geometry [65.945(16)°], the central zinc center binds to two ortho-C of two fluoroaryl groups occupying the position previously filled by a H atom and completes its tetrahedral coordination by binding to two O alkoxide atoms. In contrast, each K coordinates to the two alkoxide bridges and forms a K−F contact with one F atom ortho to the zincated C atom (Figure 1). Coordinative saturation of K is achieved by two THF molecules, one of which acts as a bridge between two K centers. While this coordination mode is rare, Strohmman recently proposed that for Lochmann-Schlosser superbase mixtures, the THF bridge could shift between the K centers providing a coordination site for substrates.34 1H-DOSY analysis in d8-THF supports that the mixed-metal/mixed-ligand constitution of compound 2 a observed in the solid state is retained in solution (see Supporting Information). Moreover, compound 2 a is remarkably stable in d8-THF since no benzyne formation was observed, though such decomposition reactions are common for these hypersensitive fluoroaryls once metalated.3
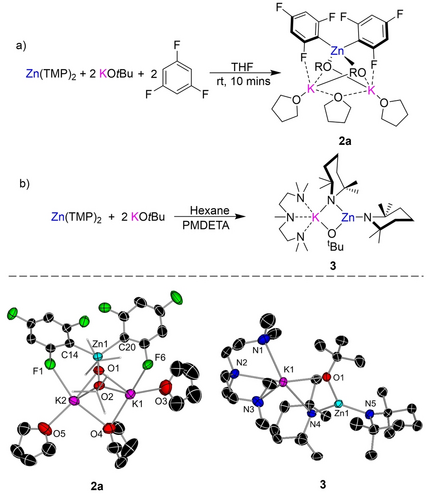
Top a) Zincation of 1,3,5-trifluorobenzene via Zn(TMP)2/2KOtBu mixture to form higher order zincate (THF)3K2Zn(C6H2F3)2(OtBu)2 (2 a). Bottom b) Formation of lower order potassium zincate (PMDETA)KZn(TMP)2OtBu (3) via co-complexation of Zn(TMP)2 and KOtBu. Molecular structures of zincates 2 a and 3 with displacement ellipsoids at 50 % probability, H atoms omitted and alkoxide C atoms drawn as wire frames for clarity.
We next evaluated the constitution of the bimetallic base by performing the reaction of 2 equivalents of KOtBu with Zn(TMP)2 in hexane in the presence of tridentate donor PMDETA (N,N,N′,N′′,N′′-pentamethyldiethylenetriamine). Despite the excess of two KOtBu employed, this reaction led to the formation of the lower order (1K : 1Zn) potassium zincate [(PMDETA)KZn(TMP)2(OtBu)] (3) as a crystalline solid in a 54 % yield. (Figure 2b). Its molecular structure consists of one potassium alkoxide unit co-complexed with Zn(TMP)2 where one TMP and the tert-butoxide group bridge between the K and Zn atoms while the remaining TMP resides terminally on Zn. PMDETA then satisfies the coordination sphere of K resulting in a structural motif previously seen in other lower order zincates.35-39 NMR spectroscopic studies suggest that this bimetallic motif is retained in d8-THF solutions with the exception of PMDETA being presumably replaced by the coordinating deuterated solvent (see Supporting Information for details). Studies assessing the co-complexation of a second equivalent of KOtBu support that in THF solution this reaction does not occur (see Supporting Information for details). This is not surprising considering the high steric demands of the TMP groups and aligns well with previous findings assessing the coordination ability of Zn(TMP)2 which fails to form adducts with donors such as pyrimidine or undergo co-complexation with Li(TMP).40, 41 In fact, a search of the CCDC revealed that 3 is the first structurally defined alkali-metal zincate resulting from co-complexation of Zn(TMP)2 with another Group 1 organometallic species.
To assess the role of the second KOtBu equivalent on the formation of 2 a we looked at the metalation of two equivalents of 1,3,5-trifluorobenzene 1 with lower order zincate 3. In agreement with our observations in Table 1, entry 4, 1H and 19F NMR reaction monitoring indicated the formation of up to four different organometallic species containing metalated aryl fragments with the full conversion of 1 and concomitant liberation of two equivalents of TMP(H). One of these species can be assigned to higher order zincate 2 a which can actually be crystallized from this reaction mixture as a minor product. Unfortunately, the other zincated products could not be identified although the disproportionation to a potassiated aryl species can be ruled out as no decomposition and/or benzyne formation with concomitant KF elimination is observed. However, as mentioned above, the complexity of this reaction mixture can be simplified by the addition of a second equivalent of KOtBu (pre or post metalation) affording higher order potassium zincate 2 a as the sole zincated intermediate (Scheme 1). These findings provide another enlightening example of the complexity of using group 1 metal alkoxides as additives to other organometallics which may not only have an activating effect but can also instigate ligand redistribution processes, affecting the speciation of the organometallic product(s).
Role of second equivalent of KOtBu in metalation of two equivalents of 1,3,5-trifluorobenzene 1 with lower-order potassium zincate 3.
Encouraged by the extremely fast and efficient zincation of 1, at room temperature using the Zn(TMP)2/2 KOtBu combination we then looked to expand the substrate scope of this approach (Figure 3). Considering the thermal stability of 2 a, reactions were carried out in THF and the zincated products were isolated as pure white solids by precipitation with hexane. Both polyfluorinated fluoroarenes 1,2,4,5-tetrafluorobenzene and 1,2,3,4,5-pentafluorobenzene were zincated to give higher-order potassium zincates 2 b and 2 c in 10 minutes at room temperature. Remarkably, despite the fragility of these sensitive fluoroaryls, 2 b and 2 c are stable at room temperature without observing any apparent decomposition after several hours in THF-d8 solutions. This thermal stability contrasts with previous zincation studies by Mulvey and Uchiyama were the products of ortho-zincation of chloro- and bromo arenes using lithium zincates can undergo rapid LiCl or LiBr elimination forming benzyne intermediates.42-44 Significantly less activated substrates such as fluorobenzene and 1-fluoronaphthalene are also quantitatively zincated in 24 h at room temperature to give products 2 d and 2 e. This is striking as previous approaches using alternative zincating reagents mentioned above fail to metalate these substrates in reasonable yields even when harsh reaction conditions are employed.28, 45
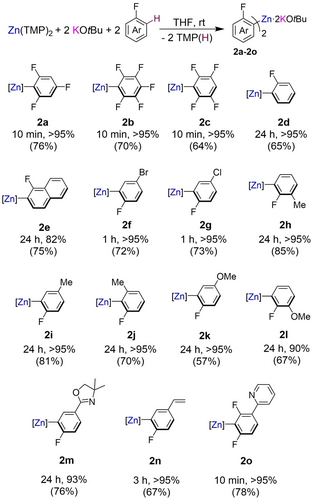
Scope of the C−H zincation of fluoroarenes using 2 : 1 mixture of KOtBu/Zn(TMP)2. NMR yields for [K2ZnAr2(OtBu)2] (2 a–2 o) determined by 1H NMR spectroscopy using hexamethylbenzene as an internal standard and isolated crystalline yields shown in brackets.
Bromo and chloro para-substituted arenes are zincated easily in 1 h to give zincates 2 f and 2 g. Ortho, meta, and para fluorotoluene can be regioselectively zincated ortho to the fluorine substituent to give products 2 h–2 j avoiding any competing lateral metalation of the methyl substituent as reported for 2-fluorotoluene using the aforementioned LiNK superbase, which favors the benzylic metalation product.7 Reactivity studies with 2- and 4-fluoroanisole revealed that acidifying effects take prevalence over coordination effects, thus favoring quantitative zincation at the ortho-position to the F substituent furnishing 2 k and 2 l (Figure 3). This preferred regioselectivity has been previously noted using Lochman-Schlosser superbases although reactions need to be performed at −78 °C. The same preference on regioselectivity was observed for 2-(4-fluorophenyl)-4,4-dimethyl-4,5-dihydroxazole where oxazoline bearing zincate 2 m was formed in a 93 % yield with regioselective zincation occurring ortho to the fluoro substituent. Knochel recently reported metalation of this substrate using a magnesium amide, which occurs preferentially ortho to the oxazoline unit, a substituent typically regarded as a very strong ortho-directing group in DoM chemistry.46
The regioselectivity of these reactions was confirmed by the structural elucidation of the molecular structures of 2 k and 2 m which display almost identical structural motifs to that described for 2 a with K preferentially interacting with the ortho-F to the C which has undergone zincation (Figure 4 and Figure S1 in Supporting Information). Olefinic groups are also tolerated as a functional group as shown by the efficient zincation of 4-fluorostyrene in 3 h furnishing 2 n. While attempts to promote regioselective zincation of fluoropyridines at room temperature proved fruitless forming intractable black solids which could not be analyzed, pyridine is tolerated as a remote functional group under these conditions as shown by the quantitative metalation of 2-(2,4-difluorophenyl)pyridine yielding zincate 2 o.
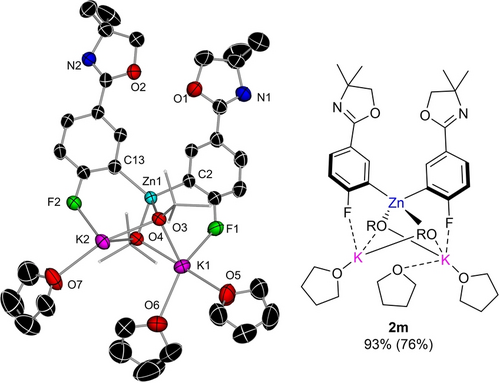
Left-hand side (LHS) – Molecular structure of zincate 2 m (LHS) with displacement ellipsoids at 50 % probability, H atoms omitted and alkoxide C atoms drawn as wire frames for clarity: (RHS) ChemDraw representation of zincate 2 m (right).
Next, electrophilic interception studies were carried out using potassium zincate 2 d, resulting from ortho-zincation of fluorobenzene (Scheme 2). Reaction with equimolar amounts of TMSCl afforded silylated species 4 in a quantitative yield. 2 d was found to participate in a copper catalysed allylation using allyl bromide to give allylated product 5 in a 62 % yield. Similarly, a copper-catalysed aroylation using benzoyl chloride gave 2-fluorobenzophenone 6 in an 88 % yield. Finally, 2 d undergoes the classical Pd catalysed Negishi cross-coupling reaction with 4-iodobenzonitrile to give bis-aryl compound 7 in a 73 % yield displaying the synthetic utility of these zincated fluoroaryl intermediates.

Electrophilic interception studies of zincate 2 d prepared in situ via zincation of 2 equivalents of fluorobenzene with a 2 : 1 mixture of KOtBu/Zn(TMP)2. [a] NMR yields determined by 1H NMR spectroscopic data using hexamethylbenzene as internal standard.
Confirming the strong metalating power of this mixed potassium/zinc bimetallic combination, we also noted that a 2 : 1 mixture of KOtBu/Zn(TMP)2 left to stir at room temperature in toluene caused the solution to turn bright orange in a characteristic trait for benzylic metalation of the arene.47 Indeed, a crop of crystals grown from the solution revealed the formation of compound [(THF)2K2Zn(Bz)2(OtBu)2]∞ (Bz=CH2Ph) (8) in a 65 % isolated yield (Figure 5).
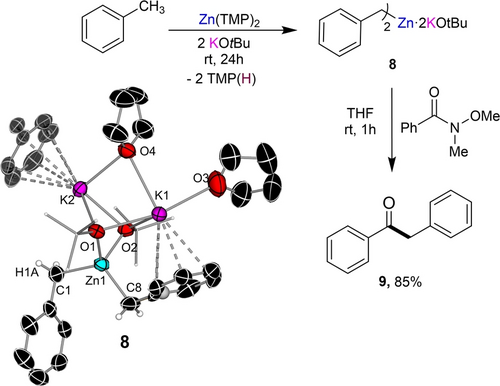
Aroylation of toluene using Weinreb amide PhC(=O)NMe(OMe) using a 2 : 1 mixture of KOtBu/Zn(TMP)2. A dinuclear unit of the polymeric structure of 8 with displacement ellipsoids at 50 % probability, H atoms omitted apart from benzylic ones and alkoxide C atoms drawn as wire frames for clarity.
X-ray crystallographic analysis revealed the formation of a polymeric higher-order potassium zincate resulting from lateral metalation of two equivalents of toluene and liberation of 2 equivalents of TMP(H). The central Zn atom bonds to two benzylic carbon anions and to two tert-butoxide anions which bridge to the two potassium atoms present. The two potassium centers connect by two bridging alkoxide groups and a bridging THF molecule. K1 completes its coordination sphere binding to a terminal THF and π-engaging in an η4-fashion with the aromatic groups of one of the benzyl anions. Similar π-interactions are observed between K2 and a benzyl group of a neighboring unit propagating the two-dimensional polymeric structure of 8 (Figure S3).48 1H and 13C NMR studies support that the bimetallic constitution of 8 is retained in solution, displaying a distinct signal at 1.67 ppm for the CH2 group in the 1H NMR spectrum.
Potassium zincate 8 can react efficiently with the Weinreb amide N-methoxy-N-methylbenzamide to give 1,2-diphenylethanone 9 in an 85 % yield. This efficiency under extremely mild reaction conditions contrasts with the inertness of both monometallic components KOtBu and Zn(TMP)2 towards this non-activated arene. As mentioned previously, the zinc amide is sold commercially as a 0.5 M solution in toluene. Remarkably, even at refluxing temperatures this benzylic zincation of toluene cannot be achieved using the lighter alkali metal alkoxide congeners LiOtBu and NaOtBu in combination with Zn(TMP)2 demonstrating a remarkable alkali-metal effect. These findings align well with recent studies which have highlighted major differences in reactivities between Li and Na and their heavier congeners, where different degrees of metal cation-π interactions have thought to be a key factor.49 As mentioned above in 8 both potassium centers π-engage with the aromatic rings of toluene. This coordination could also happen pre-metalation with the K centers acting as built-in Lewis acid centers, activating the arene towards its benzylic zincation. Interestingly this approach also worked for selective zincation of benzene, which is even less activated than toluene in terms of pKa, furnishing (THF)2K2Zn(Ph)2(OtBu)2 (10) in a 91 % isolated yield. 10 was fully spectroscopically characterized in d8-THF, including by DOSY NMR, which confirmed that its bimetallic constitution remains in solution.
Conclusion
To conclude, combining the commercially available reagents Zn(TMP)2 and KOtBu led to a profound enhancement in the reactivity of the former towards fluoroarene deprotonation reactions via the formation of a novel mixed amido/alkoxy potassium zincate. Detailed structural and NMR spectroscopic studies established the equivalency of the alkali metal alkoxide used can alter the nature of the reactive intermediates. Whilst the synthetic utility of Zn(TMP)2 has been enhanced, this work also highlights the hidden complexity on the nature of the organometallic intermediates involved. It also underlines the importance of alkali-metal effects when using alkali metal alkoxides as additives in deprotonative metalation reactions, with the K system outperforming its lighter Li and Na congeners. The potential of this bimetallic combination to promote the zincation of non-activated substrates has been demonstrated for toluene and benzene and will be further investigated in the near future.
Acknowledgments
We thank the X-ray crystal structure service unit at the University of Bern for measuring, solving, refining, and summarising the structure of compounds 2 a, 2 k, 2 m, 3 and 8.50 Swiss National Science Foundation (SNF) (projects numbers 206021_177033, 206021_198127 and 188573) and University of Bern are acknowledged for the funding of this research. Our thanks are extended to Professor Robert E. Mulvey at the University of Strathclyde for insightful discussions. Open Access funding provided by Universität Bern.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.