Facile Access to Cyclopropylboronates via Stereospecific Deborylative Cyclization: A Leaving Group-Assisted Activation of Geminal Diborons
Xin-Yi Chen
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N Zhongshan Road, Shanghai, 200062 China
These authors contributed equally to this work.
Search for more papers by this authorFeng-Chen Gao
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N Zhongshan Road, Shanghai, 200062 China
These authors contributed equally to this work.
Search for more papers by this authorPeng-Fei Ning
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorYi Wei
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Kai Hong
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N Zhongshan Road, Shanghai, 200062 China
Shanghai Frontiers Science Center of Molecule Intelligent Syntheses, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorXin-Yi Chen
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N Zhongshan Road, Shanghai, 200062 China
These authors contributed equally to this work.
Search for more papers by this authorFeng-Chen Gao
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N Zhongshan Road, Shanghai, 200062 China
These authors contributed equally to this work.
Search for more papers by this authorPeng-Fei Ning
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorYi Wei
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Kai Hong
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N Zhongshan Road, Shanghai, 200062 China
Shanghai Frontiers Science Center of Molecule Intelligent Syntheses, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorGraphical Abstract
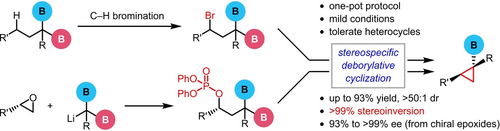
A transition metal-free deborylative cyclization strategy led to the efficient synthesis of racemic and enantioenriched cyclopropylboronates. The cyclization of geminal-bis(boronates) bearing a leaving group was highly diastereoselective and stereospecific, tolerating various functional groups and heterocycles. Mechanistic studies indicated that the leaving group at the γ-position significantly promoted the activation of the gem-diboron moiety.
Abstract
Herein we reported a transition metal-free deborylative cyclization strategy, based on which two routes have been developed, generating racemic and enantioenriched cyclopropylboronates. The cyclization of geminal-bis(boronates) bearing a leaving group was highly diastereoselective, tolerating a few functional groups and applicable to heterocycles. When optically active epoxides were used as the starting materials, enantioenriched cyclopropylboronates could be efficiently prepared with >99 % stereospecificity. Mechanistic studies showed that the leaving group at the γ-position played a crucial role and significantly promoted the activation of the gem-diboron moiety.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202302638-sup-0001-CCDC_2216460.cif1 MB | Supporting Information |
| anie202302638-sup-0001-CCDC_2216466.cif635.8 KB | Supporting Information |
| anie202302638-sup-0001-misc_information.pdf19.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. Faust, Angew. Chem. Int. Ed. 2001, 40, 2251–2253;
10.1002/1521-3773(20010618)40:12<2251::AID-ANIE2251>3.0.CO;2-R CAS PubMed Web of Science® Google Scholar
- 1bJ. Pietruszka, Chem. Rev. 2003, 103, 1051–1070;
- 1cL. A. Wessjohann, W. Brandt, T. Thiemann, Chem. Rev. 2003, 103, 1625–1648.
- 2
- 2aT. T. Talele, J. Med. Chem. 2016, 59, 8712–8756;
- 2bX. J. Dai, Y. Liu, X. P. Xiong, L. P. Xue, Y. C. Zheng, H. M. Liu, J. Med. Chem. 2020, 63, 14197–14215;
- 2cT. Maes, C. Mascaro, I. Tirapu, A. Estiarte, F. Ciceri, S. Lunardi, N. Guibourt, A. Perdones, M. Lufino, T. Somervaille, D. Wiseman, C. Duy, A. Melnick, C. Willekens, A. Ortega, M. Martinell, N. Valls, G. Kurz, M. Fyfe, J. Castro-Palomino, C. Buesa, Cancer Cell 2018, 33, 495–511.
- 3
- 3a Small Ring Compounds in Organic Synthesis VI (Ed: A. de Meijere), Topics in Current Chemistry, Vol. 207, Springer, Berlin, 2000;
10.1007/3-540-48255-5 Google Scholar
- 3bH. Lebel, J.-F. Marcoux, C. Molinaro, A. B. Charette, Chem. Rev. 2003, 103, 977–1050;
- 3cD. Y.-K. Chen, R. H. Pouwer, J.-A. Richard, Chem. Soc. Rev. 2012, 41, 4631–4642;
- 3dC. Ebner, E. M. Carreira, Chem. Rev. 2017, 117, 11651–11679;
- 3eL. Dian, I. Marek, Chem. Rev. 2018, 118, 8415–8434;
- 3fY. Cohen, A. Cohen, I. Marek, Chem. Rev. 2021, 121, 140–161.
- 4
- 4aC. Qin, V. Boyarskikh, J. H. Hansen, K. I. Hardcastle, D. G. Musaev, H. M. L. Davies, J. Am. Chem. Soc. 2011, 133, 19198–19204;
- 4bK. Liao, S. Negretti, D. G. Musaev, J. Bacsa, H. M. L. Davies, Nature 2016, 533, 230–234;
- 4cK. Liao, Y.-F. Yang, Y. Li, J. N. Sanders, K. N. Houk, D. G. Musaev, H. M. L. Davies, Nat. Chem. 2018, 10, 1048–1055.
- 5
- 5aH. C. Brown, B. Singaram, Acc. Chem. Res. 1988, 21, 287–293;
- 5bN. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457–2483;
- 5cA. J. J. Lennoxa, G. C. Lloyd-Jones, Chem. Soc. Rev. 2014, 43, 412–443;
- 5d Synthesis and Application of Organoboron Compounds (Eds: E. Fernández, A. Whiting), Topics in Organometallic Chemistry, Vol. 49, Springer, Cham, 2015;
10.1007/978-3-319-13054-5 Google Scholar
- 5eC. Sandford, V. K. Aggarwal, Chem. Commun. 2017, 53, 5481–5494;
- 5fC. Diner, K. J. Szabó, J. Am. Chem. Soc. 2017, 139, 2–14;
- 5gJ. W. B. Fyfe, A. J. B. Watson, Chem 2017, 3, 31–55;
- 5hJ. P. G. Rygus, C. M. Crudden, J. Am. Chem. Soc. 2017, 139, 18124–18137;
- 5iS. Namirembe, J. P. Morken, Chem. Soc. Rev. 2019, 48, 3464–3474;
- 5jD. M. Volochnyuk, A. O. Gorlova, O. O. Grygorenko, Chem. Eur. J. 2021, 27, 15277–15326;
- 5kO. O. Grygorenko, D. M. Volochnyuk, B. V. Vashchenko, Eur. J. Org. Chem. 2021, 6478–6510.
- 6
- 6aH. Ito, Y. Kosaka, K. Nonoyama, Y. Sasaki, M. Sawamura, Angew. Chem. Int. Ed. 2008, 47, 7424–7427;
- 6bC. Zhong, S. Kunii, Y. Kosaka, M. Sawamura, H. Ito, J. Am. Chem. Soc. 2010, 132, 11440–11442;
- 6cY. Liu, Y. Zhou, D. Li, H. Chen, J. Zhao, J. Qu, Org. Chem. Front. 2019, 6, 983–988;
- 6dL. Amenós, L. Trulli, L. Nóvoa, A. Parra, M. Tortosa, Angew. Chem. Int. Ed. 2019, 58, 3188–3192;
- 6eH. Iwamoto, Y. Ozawa, Y. Hayashi, T. Imamoto, H. Ito, J. Am. Chem. Soc. 2022, 144, 10483–10494; For the synthesis of cyclopropylboronates from alkenes and CO, see:
- 6fF.-P. Wu, X. Luo, U. Radius, T. B. Marder, X.-F. Wu, J. Am. Chem. Soc. 2020, 142, 14074–14079;
- 6gH.-Q. Geng, W. Li, Y. Zhao, X.-F. Wu, Org. Chem. Front. 2022, 9, 4943–4948.
- 7
- 7aM. Rubina, M. Rubin, V. Gevorgyan, J. Am. Chem. Soc. 2003, 125, 7198–7199;
- 7bB. Tian, Q. Liu, X. Tong, P. Tian, G.-Q. Lin, Org. Chem. Front. 2014, 1, 1116–1122;
- 7cA. Parra, L. Amenós, M. Guisán-Ceinos, A. López, J. L. García Ruano, M. Tortosa, J. Am. Chem. Soc. 2014, 136, 15833–15836;
- 7dA. Edwards, M. Rubina, M. Rubin, Chem. Eur. J. 2018, 24, 1394–1403;
- 7eX. Zhao, S. Xu, J. He, Y. Zhou, S. Cao, Org. Chem. Front. 2019, 6, 2539–2543;
- 7fA. U. Augustin, S. Di Silvio, I. Marek, J. Am. Chem. Soc. 2022, 144, 16298–16302.
- 8
- 8aT. Imai, H. Mineta, S. Nishida, J. Org. Chem. 1990, 55, 4986–4988;
- 8bS.-M. Zhou, M.-Z. Deng, L.-J. Xia, M.-H. Tang, Angew. Chem. Int. Ed. 1998, 37, 2845–2847;
10.1002/(SICI)1521-3773(19981102)37:20<2845::AID-ANIE2845>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 8cJ. E. A. Luithle, J. Pietruszka, J. Org. Chem. 1999, 64, 8287–8297;
- 8dJ. E. A. Luithle, J. Pietruszka, J. Org. Chem. 2000, 65, 9194–9200;
- 8eM. M. Hussain, H. Li, N. Hussain, M. Ureña, P. J. Carroll, P. J. Walsh, J. Am. Chem. Soc. 2009, 131, 6516–6524;
- 8fH. Lin, L. Tian, I. J. Krauss, J. Am. Chem. Soc. 2015, 137, 13176–13182;
- 8gJ. Carreras, A. Caballero, P. J. Pérez, Angew. Chem. Int. Ed. 2018, 57, 2334–2338;
- 8hB. J. Wittmann, A. M. Knight, J. L. Hofstra, S. E. Reisman, S. B. J. Kan, F. H. Arnold, ACS Catal. 2020, 10, 7112–7116;
- 8iJ. Altarejos, D. Sucunza, J. J. Vaquero, J. Carreras, Org. Lett. 2021, 23, 6174–6178;
- 8jÁ. Gutiérrez-Bonet, S. Popov, M. H. Emmert, J. M. E. Hughes, A. F. Nolting, S. Ruccolo, Y. Wang, Org. Lett. 2022, 24, 3455–3460;
- 8kM. Mali, G. V. M. Sharma, S. Ghosh, T. Roisnel, B. Carboni, F. Berrée, J. Org. Chem. 2022, 87, 7649–7657.
- 9
- 9aG. Benoit, A. B. Charette, J. Am. Chem. Soc. 2017, 139, 1364–1367;
- 9bM. Murai, C. Mizuta, R. Taniguchi, K. Takai, Org. Lett. 2017, 19, 6104–6107;
- 9cM. Sayes, G. Benoit, A. B. Charette, Angew. Chem. Int. Ed. 2018, 57, 13514–13518;
- 9dT. Ohtani, Y. Tsuchiya, D. Uraguchi, T. Ooi, Org. Chem. Front. 2019, 6, 1734–1737;
- 9eS.-S. Luo, H. Shen, S.-J. Li, T. Cao, Y.-P. Luo, S. Zhang, T. Zhou, X.-W. Liu, Org. Chem. Front. 2022, 9, 2627–2633.
- 10
- 10aI. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy, J. F. Hartwig, Chem. Rev. 2010, 110, 890–931;
- 10bJ. F. Hartwig, Chem. Soc. Rev. 2011, 40, 1992–2002;
- 10cA. Ros, R. Fernandez, J. M. Lassaletta, Chem. Soc. Rev. 2014, 43, 3229–3243.
- 11
- 11aC. W. Liskey, J. F. Hartwig, J. Am. Chem. Soc. 2013, 135, 3375–3378;
- 11bR. Murakami, K. Tsunoda, T. Iwai, M. Sawamura, Chem. Eur. J. 2014, 20, 13127–13131;
- 11cS. Miyamura, M. Araki, T. Suzuki, J. Yamaguchi, K. Itami, Angew. Chem. Int. Ed. 2015, 54, 846–851;
- 11dJ. He, H. Jiang, R. Takise, R.-Y. Zhu, G. Chen, H.-X. Dai, T. G. M. Dhar, J. Shi, H. Zhang, P. T. W. Cheng, J.-Q. Yu, Angew. Chem. Int. Ed. 2016, 55, 785–789;
- 11eJ. He, Q. Shao, Q. Wu, J.-Q. Yu, J. Am. Chem. Soc. 2017, 139, 3344–3347;
- 11fY. Shi, Q. Gao, S. Xu, J. Am. Chem. Soc. 2019, 141, 10599–10604;
- 11gY. Shi, Y. Yang, S. Xu, Angew. Chem. Int. Ed. 2022, 61, e202201463;
- 11hQ. Gao, S. Xu, Angew. Chem. Int. Ed. 2023, 62, e202218025;
- 11iT. Xie, L. Chen, Z. Shen, S. Xu, Angew. Chem. Int. Ed. 2023, 62, e202300199.
- 12For reviews, see:
- 12aN. Miralles, R. J. Maza, E. Fernández, Adv. Synth. Catal. 2018, 360, 1306–1327;
- 12bR. Nallagonda, K. Padala, A. Masarwa, Org. Biomol. Chem. 2018, 16, 1050–1064;
- 12cC. Wu, J. Wang, Tetrahedron Lett. 2018, 59, 2128–2140;
- 12dA. B. Cuenca, E. Fernández, Chem. Soc. Rev. 2021, 50, 72–86;
- 12eW. Jo, J. H. Lee, S. H. Cho, Chem. Commun. 2021, 57, 4346–4353;
- 12fC. Zhang, W. Hu, J. P. Morken, ACS Catal. 2021, 11, 10660–10680;
- 12gY. Lee, S. Han, S. H. Cho, Acc. Chem. Res. 2021, 54, 3917–3929.
- 13For transition metal-free deborylative transformations of gem-bis(boronates), see:
- 13aA. J. Wommack, J. S. Kingsbury, Tetrahedron Lett. 2014, 55, 3163–3166;
- 13bK. Hong, X. Liu, J. P. Morken, J. Am. Chem. Soc. 2014, 136, 10581–10584;
- 13cJ. R. Coombs, L. Zhang, J. P. Morken, J. Am. Chem. Soc. 2014, 136, 16140–16143;
- 13dW. Jo, J. Kim, S. Choi, S. H. Cho, Angew. Chem. Int. Ed. 2016, 55, 9690–9694;
- 13eX. Liu, T. M. Deaton, F. Haeffner, J. P. Morken, Angew. Chem. Int. Ed. 2017, 56, 11485–11489;
- 13fC. Hwang, W. Jo, S. H. Cho, Chem. Commun. 2017, 53, 7573–7576;
- 13gW. Sun, L. Wang, C. Xia, C. Liu, Angew. Chem. Int. Ed. 2018, 57, 5501–5505;
- 13hP. Zheng, Y. Zhai, X. Zhao, T. Xu, Chem. Commun. 2018, 54, 13375–13378;
- 13iP. Zheng, Y. Zhai, X. Zhao, T. Xu, Org. Lett. 2019, 21, 393–396;
- 13jW. Sun, L. Wang, Y. Hu, X. Wu, C. Xia, C. Liu, Nat. Commun. 2020, 11, 3113;
- 13kW. Jo, S.-Y. Baek, C. Hwang, J. Heo, M.-H. Baik, S. H. Cho, J. Am. Chem. Soc. 2020, 142, 13235–13245.
- 14For hydroxide-activated gem-bis(boronates) in cross-coupling, see:
- 14aK. Endo, T. Ohkubo, M. Hirokami, T. Shibata, J. Am. Chem. Soc. 2010, 132, 11033–11035;
- 14bK. Endo, T. Ohkubo, T. Shibata, Org. Lett. 2011, 13, 3368–3371;
- 14cK. Endo, T. Ohkubo, T. Ishioka, T. Shibata, J. Org. Chem. 2012, 77, 4826–4831;
- 14dC. Sun, B. Potter, J. P. Morken, J. Am. Chem. Soc. 2014, 136, 6534–6537;
- 14eB. Potter, A. A. Szymaniak, E. K. Edelstein, J. P. Morken, J. Am. Chem. Soc. 2014, 136, 17918–17921;
- 14fH. Li, Z. Zhang, X. Shangguan, S. Huang, J. Chen, Y. Zhang, J. Wang, Angew. Chem. Int. Ed. 2014, 53, 11921–11925;
- 14gL.-C. Cui, Z.-Q. Zhang, X. Lu, B. Xiao, Y. Fu, RSC Adv. 2016, 6, 51932–51935.
- 15C. Djerassi, Chem. Rev. 1948, 43, 271–317.
- 16The silica gel needs to be dried in an oven overnight prior to use to obtain good yield.
- 17R. E. Pearson, J. C. Martin, J. Am. Chem. Soc. 1963, 85, 354–355.
- 18
- 18aA. Ebrahim-Alkhalil, Z.-Q. Zhang, T.-J. Gong, W. Su, X.-Y. Lu, B. Xiao, Y. Fu, Chem. Commun. 2016, 52, 4891–4893;
- 18bS. A. Murray, M. Z. Liang, S. J. Meek, J. Am. Chem. Soc. 2017, 139, 14061–14064;
- 18cN. Miralles, J. E. Gómez, A. W. Kleij, E. Fernández, Org. Lett. 2017, 19, 6096–6099;
- 18dS. A. Murray, E. C. M. Luc, S. J. Meek, Org. Lett. 2018, 20, 469–472;
- 18eR. Gava, E. Fernández, Chem. Eur. J. 2019, 25, 8013–8017;
- 18fT. R. McDonald, S. A. L. Rousseaux, Chem. Sci. 2023, 14, 963–969.
- 19
- 19aT. Katsuki, K. B. Sharpless, J. Am. Chem. Soc. 1980, 102, 5974–5976;
- 19bK. B. Sharpless, Angew. Chem. Int. Ed. 2002, 41, 2024–2032;
10.1002/1521-3773(20020617)41:12<2024::AID-ANIE2024>3.0.CO;2-O CAS PubMed Web of Science® Google Scholar
- 19cM. Tokunaga, J. F. Larrow, F. Kakiuchi, E. N. Jacobsen, Science 1997, 277, 936–938;
- 19dS. E. Schaus, B. D. Brandes, J. F. Larrow, M. Tokunaga, K. B. Hansen, A. E. Gould, M. E. Furrow, E. N. Jacobsen, J. Am. Chem. Soc. 2002, 124, 1307–1315;
- 19eT. Hamada, T. Torii, K. Izawa, R. Noyori, T. Ikariya, Org. Lett. 2002, 4, 4373–4376.
- 20A. Bonet, M. Odachowski, D. Leonori, S. Essafi, V. K. Aggarwal, Nat. Chem. 2014, 6, 584–589.
- 21
- 21aG. Zweifel, H. Arzoumanian, C. C. Whitney, J. Am. Chem. Soc. 1967, 89, 3652–3653;
- 21bG. Zweifel, N. L. Polston, C. C. Whitney, J. Am. Chem. Soc. 1968, 90, 6243–6245;
- 21cD. A. Evans, T. C. Crawford, R. C. Thomas, J. A. Walker, J. Org. Chem. 1976, 41, 3947–3953;
- 21dR. P. Sonawane, V. Jheengut, C. Rabalakos, R. Larouche-Gauthier, H. K. Scott, V. K. Aggarwal, Angew. Chem. Int. Ed. 2011, 50, 3760–3763.
- 22K. M. Sadhu, D. S. Matteson, Organometallics 1985, 4, 1687–1689.
- 23
- 23aC.-T. Yang, Z.-Q. Zhang, H. Tajuddin, C.-C. Wu, J. Liang, J.-H. Liu, Y. Fu, M. Czyzewska, P. G. Steel, T. B. Marder, L. Liu, Angew. Chem. Int. Ed. 2012, 51, 528–532;
- 23bS. N. Mlynarski, C. H. Schuster, J. P. Morken, Nature 2014, 505, 386–390.
- 24V. Bagutski, T. G. Elford, V. K. Aggarwal, Angew. Chem. Int. Ed. 2011, 50, 1080–1083.
- 25E. Vedejs, R. W. Chapman, S. C. Fields, S. Lin, M. R. Schrimpf, J. Org. Chem. 1995, 60, 3020–3027.
- 26Presumably >99 % ee according to ref. 24 and the synthesis of 57.
- 27Deposition numbers 2216460 (for 9), and 2216466 (for 10) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 28We also derivatized compound 41 and compared with a commercially available authentic sample to confirm the absolute configuration. See Supporting Information for details.
- 29
- 29aH. L. Goering, S. L. Trenbeath, J. Am. Chem. Soc. 1976, 98, 5016–5017;
- 29bM. F. Hawthorne, J. A. Dupont, J. Am. Chem. Soc. 1958, 80, 5830–5832.
- 30R. Larouche-Gauthier, T. G. Elford, V. K. Aggarwal, J. Am. Chem. Soc. 2011, 133, 16794–16797.