Linear and Bent Cp*2Si: Reversible Phase Transition of a Key Molecule
Graphical Abstract
The organometallic lighthouse molecule Cp*2SiII was always the odd one out of the heavier homologues. In the solid-state counterintuitively there is a linear molecule next to two bent ones. While the latter is more stable, the linear conformer was mainly attributed to fuzzy packing effects. Now it is clear that the linearity has to be attributed to entropy in the room temperature phase while the bending is due to dispersion of the rings in the 80 K phase.
Abstract
The solid-state structure of decamethylsilicocene Cp*2Si with a bent and a linear molecule in the same unit cell was so far considered an exception in relation to the structures of its all-bent heavier analogues Cp*2E with E=Ge, Sn, Pb. Here, we present the solution to this conundrum by reporting a low-temperature phase, where all three symmetrically independent molecules are present in a bent formation. This reversible enantiotropic phase transition occurs in the temperature range between 80 K and 130 K and provides a rationale for the unexpected linear molecule based in entropy beyond hand-waving explanations such as electronic reasons or packing effects.
Organometallic compounds of divalent Group 14 elements played an important role in the development of bonding concepts and molecular structures. A key structural feature is a stereo-chemically active and energetically high-lying electron lone pair, which is also the centre of reactivity in these compounds. The iconic cyclopentadienyl ligand is a cornerstone in the development of stable organometallic species of divalent Group 14 elements, as π-interactions moderate the Lewis-acidity at the E-centres. Starting from pioneering Cp2E compounds an immensely rich chemistry of tetrylenes R2E has been developed in the past five decades. Today these systems have even been applied in bond activation1 or catalysis.2 Yet, despite being stabilised by the Cp-ligands, the relatively stable tetrelocenes and in particular their lightest congener, silicocene are ground-breaking textbook compounds of fundamental relevance.
In general, the field of cyclopentadienyl compounds of Group 14 elements has captured the interest of synthetic chemists since the initial report of bis(cyclopentadienyl) lead in 1956 by Fischer and Grubert3 and the report of Cp2Sn in the same year.4 In the following decades the field has been pushed forward by the synthesis of Cp2Ge.5 While the structure of Cp2Ge has been reported by Grenz et al.,6 the structure of monomeric Cp2Sn was reported by Atwood and Hunter.7 In the same publication they also reported the synthesis and solid-state structure of decamethylplumbylene. Previously, the use of pentamethylcyclopentadienyl substituents had been successfully reported by Jutzi and co-workers8 for both tin and germanium, which not only yielded the structures themselves but also enabled the synthesis of pentamethylcyclopentadienyl germanium and tin cations. Finally, following the approach published for germanium and tin earlier,9 Jutzi and co-workers managed to report the first synthesis and solid-state structure of decamethylsilicocene as the first derivative of silicon in oxidation state (II).10 Shortly after the same group conducted a detailed analysis of the compound by a plethora of analytic and theoretical methods.11
The solid-state structure as described by Jutzi, Krüger, et al. (see analogous structure in Figure 1, left) contains decamethylsilicocene molecules in two different conformations.10 The first molecule adopts the bent conformation, which is well known for the corresponding structures of germanium and tin8 and is the structure to be expected for a SiII compound with a supposedly stereochemically active electron lone pair residing at the silicon atom. Only half of the second molecule is contained in the crystallographic asymmetric unit, whereas the other half is generated by an inversion centre. Accordingly, this molecule conformation is a D5d symmetric heel-and-toe sandwich complex with plane-parallel cyclopentadienyl rings. Overall, there are twelve molecules in the unit cell, from which four adopt linear and eight adopt bent conformation.
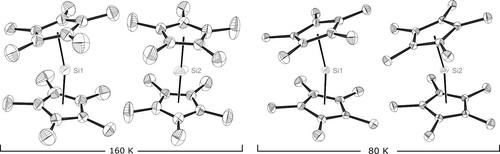
Molecules in the crystal structure of decamethylsilicocene at 80 K and 160 K. The third symmetrically molecule in the asymmetric unit of the 80 K was omitted as it is very similar to the left structure within the respective block.
The presence of two distinct conformers was explained by the original authors by “intermolecular interactions and packing effects” causing the bent conformer. While the Jutzi group could successfully show that the bent conformer is the most stable in the gas phase,11 packing effects kept being mentioned in subsequent publications12 and were used to explain differences between a calculated bent structure of Cp2Si and the experimental structure of Cp*2Si.13
The presence of two very distinct conformers with a differing nature of the respective SiII lone-pairs prompted the measurement of a high-quality X-ray diffraction dataset at 90 K14 with the original purpose of an experimental determination of the electron density. Ultimately, the large degree of flexibility in the molecule limited the resolution to 0.60 Å and therefore impeded such an investigation. However, the determined structure showed a new phase in a different space group. Subsequently, we want to resolve the seemingly unique feature of decamethylsilicocene by reporting a non-destructive phase transition, with only bent decamethylsilicocene molecules in the unit cell at low temperatures (Figure 1, right). To further characterise the phase transition, we have collected additional single crystal X-ray diffraction data for temperatures between 80 K and 160 K to investigate the phase transition from the previously reported high temperature C2/c phase to the low temperature phase, best described in P21/c (For the detailed sequence of investigated temperatures, see the Supporting Information).
The most obvious approach is to trace the change in geometry, i.e. the change of the tilt angle α defined as the angle between the silicon atom and its projection onto either of the cyclopentadienyl C5-planes. Consequently, we refined all collected diffraction data in P21/c. The resulting angles are depicted in Figure 2 in addition to plane-wave DFT calculations for comparison (see below).
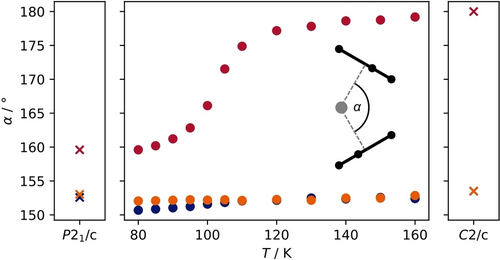
Development of the bent angle α for the three independent molecules found in the asymmetric unit of the P21/c low temperature phase of Cp*2Si with increasing temperature (circles) and the calculated angles for the theoretical calculations of the two different structures in their respective space groups (crosses).
The plane-wave DFT calculations using ultra-soft pseudo-potentials were conducted using Quantum Espresso15 and the PBE16 functional and cut-off energies of 80 Ry for the wavefunction and 800 Ry for the density at the Γ-point, as well as Grimme's D3BJ17, 18 correction for dispersive interactions. For both structures, atomic positions and the size of the unit cell were optimised starting from the experimental geometries with the respective symmetry elements of the space group enforced during the calculation. As expected from the presence of the two phases, the relative energies are similar with slightly lower calculated energy for the low temperature phase. The calculated difference is 0.191 eV for the two complete unit cells, which corresponds to 1.54 kJ mol−1 for Cp*2Si with 12 molecules per unit cell.
Obviously the two molecules that are bent (and related by crystallographic symmetry) in the high temperature phase, slightly deviate from each other in the low temperature phase. Starting from α=152.60(12)° (calc 153.50°), the first angle narrows upon cooling by almost 2° to 150.69(11)° (calc. 152.57°) while the second shows a much smaller deviation of about 0.4° to 152.05(11)° (calc 153.03°) at 80 K. As expected, the effect is much more pronounced for the linear molecule, where the angle starts at 180° at 160 K (180° in calculation) and changes to 159.60(12)° (calc 159.60°) at 80 K, thereby not reaching the angles exhibited by the other two bent molecules in the asymmetric unit.
The size of the unit cell parameters a and b shows plateaus between 95 K and 110 K, while the slope of the increase of unit cell parameter c with temperature changes at around 110 K. For a detailed plot and the corresponding numbers see the Supporting Information Figure S3 and Table S2. The phase transition can also be traced by the difference in systematic absences (Figure 3), as the space group changes from C2/c to P21/c. The emergence of the previously absent reflections upon cooling can clearly be traced between 120 K and 90 K. The intensity of reflections systematically absent for C2/c is high at 90 K with the mean I/σ being 20.3 compared to 20.9 for the reflections present in both space groups. This changes to 12.5/19.0 at 100 K. At 120 K the mean I/σ is only 0.9, which means the systematic absences are fulfilled on average. Meanwhile the remaining reflections show a mean I/σ of 15.9.
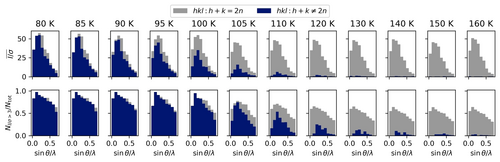
Temperature dependence of mean reflection intensity over estimated standard deviation (top) and fraction of reflections fulfilling the significance criterion (bottom) in resolution bins for the reflections present in both space groups (grey) and the reflections that are systematically absent in the high temperature space group (blue).
If both conformers are present in the solid state the question occurs, how large the energy difference between the two conformers actually is. Several authors have already weighted in on this problem. One of the most in-depth discussion was provided by Schoeller et al.19 By using CCSD(t) calculations, they could show, that Cp2Si is bent. Additionally, they concluded from a comparison to B3LYP/6-31G(d), that no essential differences are caused by the methyl groups. Sapunov et al.20 included Cp*2Si in their analysis of through-space coupling and stated that “most differences between the s0p0 and s2p0 metallocenes concern secondary, not principal, variations in the structural and reactivity patterns.” and attributed the structure in large part to the equilibrium between van-der-Waals attraction and repulsion.
As such, we wanted to contribute calculations, which include dispersion correction. We optimised the Cp*2Si employing the PBE0 functional21 using the def2-TZVP basis set22 and the D3BJ scheme proposed by Grimme et al.17, 18 for dispersion correction using the ORCA23 calculation package. The first geometry was obtained with enforced Ci symmetry, while the second was obtained without any symmetry. The bent C1 conformation yields a lower energy by about 4.5 kJ mol−1 compared to the conformation fixed to Ci symmetry. About 3.5 kJ mol−1 result from the dispersion correction. Per se, dispersion interaction plays an important role in favouring the bent formation, even if the overall differences are small. It should be noted, that the Ci conformation does not comprise a local minimum in the calculation. Instead, two vibration modes with imaginary frequencies can be observed, both of which belong to the slippage modes of the Cp* rings around the silicon atom.
While it seems plausible, that the additional dispersive interaction in the bent conformer stems from the interaction of the two Cp* groups, we still want to verify this fact. In order to localise the interaction, we used NCIPlot.24 As can be seen in Figure 4, the dispersive interaction is localised between the two Cp* substituents, further underpinning the explanation, that dispersive interactions between the two ligands are the source for the energetic difference between the two conformers, which ultimately leads to favouring the bent formation. While dispersive interactions are also present for the centrosymmetric Cp*2Si, the larger distance reduces the strength of this interaction, which is reasonable considering that van-der-Waals interactions have a dependence on the distance of r−6.
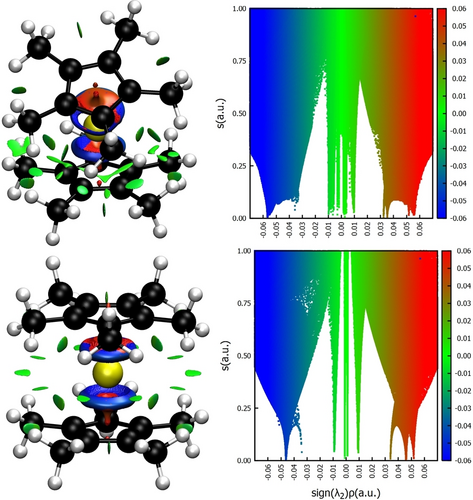
NCI Analysis24 depictions for the optimised structures of the bent conformer (top row) and the linear conformer (bottom row). Depicted is the reduced density gradient as isosurface of the level 0.3 a.u., where the green colour indicates (RDG) low density accumulation and therefore dispersive interactions. On the right values of the RDG are plotted against the sign of λ2 multiplied by the density.
In conclusion, we could successfully explain the unique position taken by Cp*2Si so far, rationalising both, the linear and the bent conformation in the solid-state and thus allowing for discussion about its electronic structure. The linear conformation of the molecule is only present in the high temperature phase, but all molecules adopt a bent conformation at low temperatures. Therefore, the driving force for the high-temperature phase with the linear conformation should be entropical, and not be searched in the energy. In fact, the linear conformation does not comprise a minimum in the employed theoretical calculations, while the periodic plane-wave calculation also shows a lower energy for three bend conformers in the solid-state structure.
We could attribute a large part of this stabilisation to dispersive interactions, even though the overall stabilisation is only small. More than 35 years passed since the initial report of the structure and this revision. We were able to profit from advancements in technology and software for X-ray diffraction measurements, especially in routine crystal cooling. As such, we could demonstrate that we should be aware of what we are actually recording. Equilibrium does not mean we are at the minimum of the electronic energy surface, especially at higher temperatures, where entropic effects play an increasingly important role. Keeping this in mind, low temperature measurements should now be routine wherever possible, especially if we want to supply generalisable statements from the obtained geometries. At the same time, we should also be cautious to attribute differences to the fuzzy concept of “packing effects”, which usually has very low predictive and no explanatory power. Revisiting structures, where packing effects were used as an explanatory last resort to “clarify” room temperature geometries, might prove worthwhile.
Acknowledgments
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—389479699/GRK2455. Computational results were obtained using the Göttingen Chemistry Compute Cluster. This project has been funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—project number 405832858 Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.