Precise Epitope Organization with Self-adjuvant Framework Nucleic Acid for Efficient COVID-19 Peptide Vaccine Construction
Graphical Abstract
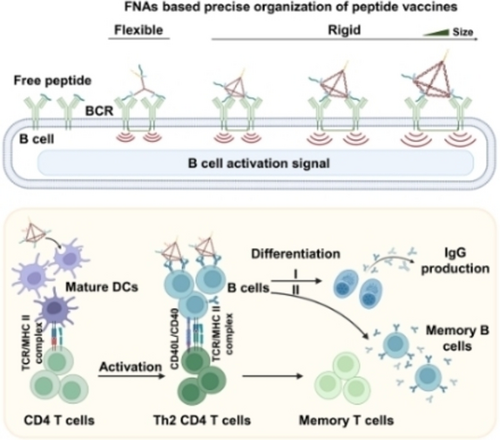
Framework nucleic acids (FNAs)-based peptide vaccines with different structures and sizes were constructed to understand the effect of peptide spacing and carrier rigidity on B cell activation, and reveal the significance of precise peptide organization. Epitopes from SARS-CoV-2 were assembled on the preferred FNA to construct a COVID-19 peptide vaccine prototype which effectively triggered humoral immune responses in mice.
Abstract
Peptide vaccines have advantages in easy fabrication and high safety, but their effectiveness is hampered by the poor immunogenicity of the epitopes themselves. Herein, we constructed a series of framework nucleic acids (FNAs) with regulated rigidity and size to precisely organize epitopes in order to reveal the influence of epitope spacing and carrier rigidity on the efficiency of peptide vaccines. We found that assembling epitopes on rigid tetrahedral FNAs (tFNAs) with the appropriate size could efficiently enhance their immunogenicity. Further, by integrating epitopes from SARS-CoV-2 on preferred tFNAs, we constructed a COVID-19 peptide vaccine which could induce high titers of IgG against the receptor binding domain (RBD) of SARS-CoV-2 spike protein and increase the ratio of memory B and T cells in mice. Considering the good biocompatibility of tFNAs, our research provides a new idea for developing efficient peptide vaccines against viruses and possibly other diseases.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.