Chemical Modification of a Bacterial Siderophore by a Competitor in Dual-Species Biofilms
Graphical Abstract
Detailed investigation of the inter-species chemical communication between the human pathogens P. aeruginosa and S. aureus revealed that S. aureus converts pyochelin, an important siderophore secreted by P. aeruginosa, to its less potent pyochelin methyl ester (PME). In addition, PME is sensed by P. aeruginosa and affects metabolite expression, indicating a potential role as signaling molecule for both species.
Abstract
Chemical communication between competing bacteria in multi-species environments often enables both species to adapt and survive, and perhaps even thrive. P. aeruginosa and S. aureus are two bacterial pathogens found in natural biofilms, especially in the lungs of cystic fibrosis (CF) patients, where recent studies showed that there is often cooperation between the two species, leading to increased disease severity and antibiotic resistance. However, the mechanisms behind this cooperation are poorly understood. In this study, we analyzed co-cultured biofilms in various settings, and we applied untargeted mass spectrometry-based metabolomics analyses, combined with synthetic validation of candidate compounds. We unexpectedly discovered that S. aureus can convert pyochelin into pyochelin methyl ester, an analogue of pyochelin with reduced affinity for iron (III). This conversion allows S. aureus to coexist more readily with P. aeruginosa and unveils a mechanism underlying the formation of robust dual-species biofilms.
Introduction
Pseudomonas aeruginosa and Staphylococcus aureus are opportunistic pathogens that exploit weaknesses in host defense systems to establish infections. They are among the leading pathogens in nosocomial infections and are commonly isolated from cystic fibrosis (CF) patients who suffer from chronic lung infections.1, 2 In the course of the disease, S. aureus is more common during childhood, and as the patient ages, P. aeruginosa becomes the dominant species.3
In planktonic cocultures, the presence of P. aeruginosa drives S. aureus towards a fermentative state and reduces viability by secretion of virulence factors such as 2-n-heptyl-4-hydroxyquinoline N-oxide (HQNO) and the siderophores pyoverdin and pyochelin.4 However, recent studies on CF isolates indicate that under certain conditions S. aureus can not only survive the presence of P. aeruginosa, but the two species can in fact thrive in a mutual biofilm. Biofilms consist of a highly organized matrix that nurtures the bacteria and protects them from outside factors, such as a host's immune system and antibiotic treatment. Such dual-species biofilms have shown to be more resistant to antibiotics than corresponding monoculture biofilms.5, 6 In this form of growth, P. aeruginosa reduces the production of HQNO and the siderophores, allowing S. aureus to coexist by adopting the small colony variant (SCV) phenotype.5, 7 Because the SCVs are hard to detect, it often leads to the misleading diagnosis of P. aeruginosa monoculture; therefore, not much is known about the interactions between the pathogens and their synergistic effect under biofilms.
One molecular mechanism that bacteria have developed to communicate with each other is called quorum sensing (QS).8 QS allows bacteria to coordinate their behavior based on the density of the population. It is usually composed of several regulatory systems in the bacteria, with each system based on a receptor and its cognate signaling molecule, called an autoinducer (AI).9, 10 Upon binding its target protein, the AI-protein complex initiates expression of certain sets of genes that regulate virulence factor production, pathogenicity, biofilm formation and expression of secretion systems (SSs) such as T3SSs and T6SSs, which facilitates the function of virulence factors.11 The secretion of these primary and secondary chemical signals depends on the growth status and environmental conditions surrounding the bacteria; as such, the metabolic profile of bacteria in planktonic cultures is markedly different from their metabolic profile inside biofilms.
In dual species biofilms, iron availability is also a key factor that influences the growth and behavior of the two bacterial species. One of the strategies for acquiring iron is by releasing siderophores to the environment. P. aeruginosa regulates production and secretion of the siderophores pyoverdin and pyochelin by QS.12, 13 Although pyoverdin has a markedly higher affinity to iron (III) than pyochelin,14 both are important tools for P. aeruginosa to import iron in challenging environments such as the human lung.15 In order to identify unknown small molecules that mediate interactions between P. aeruginosa and S. aureus in the complex environment of dual species biofilms, we decided to perform untargeted mass spectrometry-based metabolomics analyses. Significant advances in metabolomics methodologies in recent years have led to their successful use in studying complex biological systems.16 By combining multiple databases and statistical analysis tools, this approach enables the detection and characterization of specific signaling molecules that may regulate chemical communication between bacteria. In this study, we unexpectedly discovered that S. aureus can convert pyochelin into pyochelin methyl ester, an analogue of pyochelin with reduced affinity to iron (III). This conversion allows S. aureus to coexist more readily with P. aeruginosa and unveils a mechanism underlying the formation of robust dual-species biofilms.
Results and Discussion
P. aeruginosa and S. aureus can form mutual biofilms under conditions that limit virulence
We set out to find appropriate conditions that allow for coexistence of both pathogens in biofilms, and this required optimization of various parameters, such as type of medium, supplementals and various other growth conditions. While P. aeruginosa proliferates rather easily under most conditions, achieving S. aureus survival in the dual-species biofilm was more challenging. For this purpose, in a recent study Dulbecco's modified Eagle's medium (DMEM) was found to be a better medium for mutual biofilm formation as compared to LB, TSB and SCFM2.17 The authors also tested the effect of BSA and reported benefits to S. aureus proliferation, which we examined as well. In addition, we examined incubation under 5 % CO2 while the medium was supplemented with NaHCO3 to maintain a stable physiological pH. The S. aureus—P. aeruginosa inoculation ratio was 1 : 1 and we grew the biofilm samples for 72 h in order to allow establishment of S. aureus colonies and to let the inter-species signals accumulate for further analyses. We confirmed that BSA is necessary for S. aureus survival for 72 h in mutual biofilms, while in the samples without BSA no S. aureus was observed. The incubation with 5 % CO2 also enhanced S. aureus survival (Figure S1) and resulted in less variation and more reproducible results.
The metabolic profile for both species in biofilms is dependent on S. aureus inoculum size
It became clear during our optimization studies that a major factor defining the establishment of a successful mutual biofilm is the relative inoculum size of both species. Therefore, we decided to analyse the changes in metabolic output for both species as a function of inoculum size. To this end, we used untargeted metabolomics methods. We systematically examined the effects of different S. aureus (SA)—P. aeruginosa (PA) starting ratios in the mixed biofilm samples. Three different bacterial biofilms with increasing SA/PA inoculum ratios were analysed (1 : 1, 5 : 1 and 10 : 1 SA : PA, respectively). After incubation, the molecules were extracted with methanol and the samples were analysed using high-resolution tandem mass spectrometry coupled with ultra-high-performance liquid chromatography (UHPLC-HRMS/MS). The molecules in the samples are characterized by a unique retention time and MS2 fragmentation and therefore can be tracked and annotated using designated databases. The metabolome of the samples was analysed using the open-source MetaboAnalyst 5.018 and is represented by principal component analyses (PCA). The PCA plot in Figure 1A reveals that the metabolic profile of the mixed samples more resembles P. aeruginosa monocultures than S. aureus monocultures. The differences between the inoculum ratios are reflected on the PC3 axis. As the S. aureus concentration increases, the mixed biofilm's metabolic profile changes, shifting to the left on the PC3 axis, drifting away from the PA14 monoculture profile (Figure 1B). The PCA plot indicates there are differences in P. aeruginosa metabolite secretion in response to S. aureus presence.
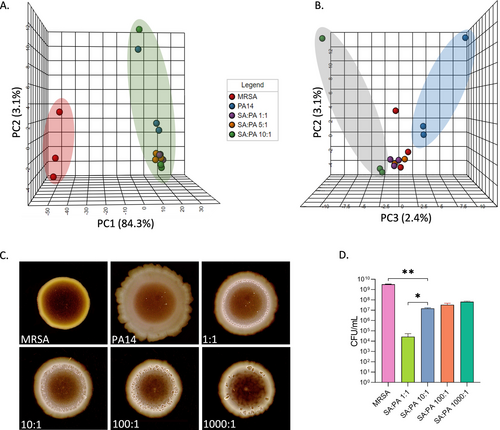
Co-cultured biofilms characterization. A) Principal component analysis score plot with PC1-PC2 axis and B) PC2-PC3 axis for comparing biofilms metabolic profile. C) Colony biofilm phenotype changes with increased S. aureus concentration. D) S. aureus viability in the dual biofilms. For A and B, n=3 after substructions of agar samples as blank. For C and D Error bars represent SD of three replicates. Data were analysed using Tukey's multiple comparisons test. *P<0.1, **P<0.01.
Increased inoculum size of S. aureus changes mutual biofilm morphology
Microbial biofilm morphologies reflect changes in microbial physiology and metabolic state and thus serve as an indicator for these changes. To evaluate these processes, we again increased the starting inoculum ratios of the mixed biofilms (1 : 1, 10 : 1, 100 : 1, and 1000 : 1 SA : PA, respectively). After 72 h of incubation, colony morphology was visualized against a black background (Figure 1C). While PA14 has a smooth morphology with a particular flower-shape at the periphery, this phenotype diminishes in the presence of MRSA. As the SA/PA inoculum ratios increase, the inner bright circle increases in size and more wrinkles appear while the smooth periphery disappears. The formed inner bright circle has the same size and shape as MRSA monocultures and it might serve as indication to the location of S. aureus, perhaps as microcolonies, inside the dual-species biofilm. Even though MRSA viability (Figure 1D) doesn't change significantly from 10 : 1 to 1000 : 1 samples, the colony morphology undergoes notable changes, strengthening our previous observations of changes in metabolic profiles (PCA plots in Figures 1A, B)—there is a change in the metabolites that are secreted in the samples as a result of S. aureus and P. aeruginosa interactions.
The concentration of a pyochelin derivative increases in the presence of S. aureus
Our next step was to identify specific metabolites that contribute to the changes in the metabolic profile discussed in the previous sections. For this purpose, data were uploaded to the Scripps XCMS online platform19 which can be used to compare relative m/z intensities between different samples. This method allowed us to detect molecules secreted by P. aeruginosa, whose abundance depends on S. aureus population density. By combining this tool with GNPS20 (The Global Natural Product Social Molecular Networking) databases, several relevant metabolites were identified. One interesting metabolite was annotated in GNPS as pyochelin methyl ester (PME), a derivative of the known siderophore pyochelin (Figure 2A). The increase in PME concentration in the mutual biofilms is correlated to S. aureus abundance in these biofilms (Figures 2B, C).
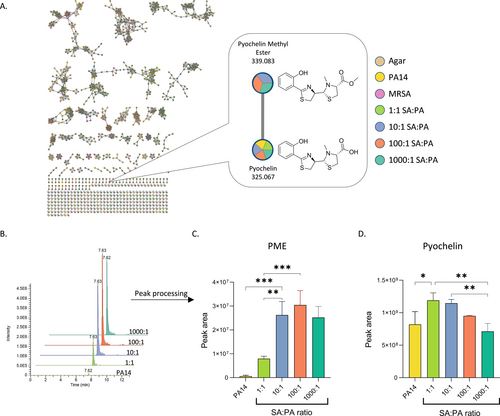
Metabolites identification and quantification. A) Molecular network of the extracted metabolites visualized by Cytoscape21 and molecules annotation by GNPS. B) Representative peak intensity chromatogram and C) calculated peak area of pyochelin methyl ester (PME) detected in the samples. D) Calculated peak area of pyochelin detected in the samples. Error bars represent SD of three biological replicates. Data were analysed using Tukey's multiple comparisons test. *P<0.1, **P<0.01, ***P<0.001.
Pyochelin is a low-molecular-weight chelating agent secreted by P. aeruginosa that scavenges iron from its surroundings and imports the complex through specific receptors.22, 23 During our mixed biofilm studies, we observed that the concentration of pyochelin consistently decreases as the content of S. aureus in the mixed biofilm increases (Figure 2D). This trend is correlated with other studies showing a reduction in siderophore production in cocultured biofilms.4, 22 The reduction of pyochelin is inversely correlated with the increasing concentrations of PME, indicating a direct or indirect relation between the two metabolites. However, additional factors contributing to pyochelin reduction might be involved as well.
Elucidation of the structure of PME
Although GNPS provided an annotation showing that the metabolite we detected is PME 1 with high confidence (a cosine score of 0.87), this annotation was suggested to be the methylated pyochelin 2 (by Olakunle et al.23) with the methyl group placed on the thiazolidine ring, on the carbon adjacent to the carboxylic acid moiety (Figure 3A).
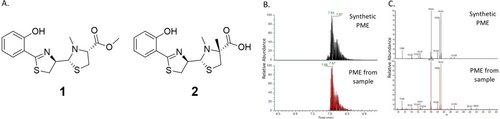
PME structure identification. A) Potential structures of the identified pyochelin derivative; PME (1), methylated pyochelin (2). B) Total ion count chromatograms for synthetic and extracted PME and C) MS2 fragmentation spectra for both samples.
As we surmised that an alternate structure of this pyochelin analogue would be more likely—i.e. a methyl ester (1)—pyochelin from P. aeruginosa cultures was extracted and purified using preparative HPLC (Figures S2, S3). Then, pyochelin was esterified to give the methyl ester form, thus creating synthetic PME 1 from the natural pyochelin (Figures S4A, B). The synthetic PME 1 was compared to the PME found in the SA:PA cocultures using UHPLC-HRMS/MS and the retention time (RT) and MS2 fragmentation were compared (Figures 3B, C). Results indicate that the synthetic PME 1 closely matches the PME that was found in the sample, as its retention time and the MS2 fragments are nearly identical. These parameters provide the highest identification, level I, as stated by the Metabolomics Molecular Standards Initiative (MSI).24 Based on the presence of a free carboxylic acid moiety in structure 2, we assumed that this compound would have a retention time similar to that of pyochelin and its enantiomer (with RT=6.11, 6.30 min, Figure S2). On the other hand, PME 1 has a retention time that is 1.2 min longer than pyochelin's RT because of the ester group. Therefore, we can determine that the correct structure for the compound identified with the help of GNPS is structure 1 and we will refer this structure as PME from here on.
Pyochelin is converted to PME by enzymatic activity of S. aureus
In order to examine how pyochelin is converted to PME, 10 μM pyochelin (extracted and purified from P. aeruginosa) was incubated with S. aureus in DMEM. In addition, S. aureus supernatants were also incubated in the presence of pyochelin at the same concentration to examine if the conversion occurs by metabolites or enzymes that are secreted by S. aureus. Additionally, pyochelin was incubated only with DMEM as a control experiment to examine if the esterification occurs spontaneously in growth media. After 17 h of incubation the samples were filtered and analysed in the UHPLC-HRMS/MS. As shown (Figure 4), out of the three conditions, PME appears only in the sample to which MRSA was added. Since S. aureus creates an acidic environment while increasing its population density, we suspected that this might play a role in the esterification of pyochelin; therefore, the conversion was tested also under buffered conditions close to neutral pH. Again, PME appeared only in sample that contained MRSA while the medium pH remained close to neutral (6.8) during and after incubation.
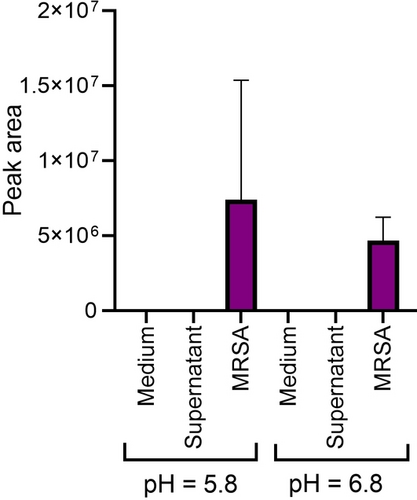
PME relative abundance in the samples. Error bars represent SD of four replicates.
These results excitingly show that S. aureus can convert pyochelin into PME. In the course of the preparation of this manuscript, Jenul et al.25 reported similar findings as well as the identification of a S. aureus enzyme (Spm) that appears to be responsible for the esterification of pyochelin. Importantly, in this study a skin wound co-infection experiment in mice was performed and a Δspm S. aureus strain showed reduced fitness in the presence of P. aeruginosa. Altogether, this research supports our findings that S. aureus contains an enzyme that is responsible for the conversion of pyochelin into its methyl ester.
PME has a reduced ability to chelate iron
The conversion of pyochelin to PME is not restricted only to P. aeruginosa and S. aureus, as one other example (in soil communities) was reported: pyochelin from Pseudomonas chlororaphis was found to be converted to PME by Bacillus amyloliquefaciens.26 In addition, pyochelin secreted by Burkholderia cenocepacia was reported to be modified to pyochelin glycolic acid by the fungus Phellinus noxius.27 In this case, the modification was found to reduce the iron chelating capacity of pyochelin. Pyochelin has been shown to chelate iron (III) in 2 : 1 ratio, where one molecule acts as a tetradentate ligand, and the second molecule is involved as a bidentate unit (Figure 5A).14, 28 We suspected that PME, similar to pyochelin glycolic acid, will also demonstrate a lower affinity to iron due to the lack of the carboxylate moiety that is involved in the iron chelation. While the free siderophore has a robust fluorescent signal, the siderophore-Fe3+ complex fluorescence is quenched. Therefore, the fluorescence of pyochelin and PME was measured in the presence of increasing iron (III) concentrations. The apparent dissociation constant for these complexes (Kd,app) represent the relative concentration at which half of the pyochelin fluorescence is quenched by the available iron. While determination of precise binding parameters (e.g. pFe) for potential PME-Fe3+ complexes at various conditions and binding modes requires more advanced analyses, these measurements do show that PME displays a lower affinity for iron (III) compared to pyochelin (Figure 5B). Although the difference in this affinity is not large, the ability of S. aureus to at least partially inactivate an important P. aeruginosa siderophore suggests that this may elevate its fitness in a dual-species biofilm. The slight difference in binding affinity motivated us to explore if there are any further advantages for S. aureus or P. aeruginosa to produce PME.
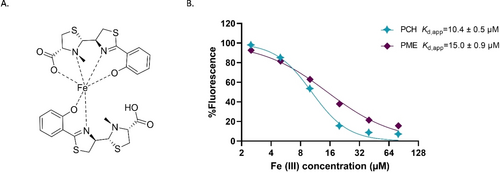
A) Pyochelin-iron complex. B) PCH [20 μM] and PME [20 μM] fluorescence quenching by Fe3+ binding, error bars (smaller than the size of the symbols) represent SD of three replicates. Curve fits and Kd,app values where calculated by nonlinear regression.
P. aeruginosa alters metabolite production as a function of PME concentration
As we discovered, PME is formed as part of the defense mechanism of S. aureus, but we hypothesized that it could also serve as a signaling molecule for P. aeruginosa. By recognizing PME, P. aeruginosa may be able to sense which species are present in its surroundings and modify its behavior accordingly, based on their population densities. In order to evaluate this hypothesis, P. aeruginosa was incubated in the presence of PME and pyochelin, as control, in different concentrations. In addition, since PME can mildly chelate iron, another control experiment was performed with increasing concentrations of EDTA, a known iron chelator, at non-toxic concentration ranges (10 μM and below, Figure S5).29, 30 This control experiment enables differentiation between the response to PME and the response towards iron starvation. The secretion of metabolites was analysed by UHPLC-HRMS/MS. As the resulting principal component analysis reveals (Figure S6), the metabolic profile of P. aeruginosa is strongly influenced by the presence of PME.
Figure 6A shows an example of changes in concentrations of a specific metabolite as a result of the increasing concentrations of PME. This metabolite does not appear either in the control experiment in which P. aeruginosa is treated with pyochelin nor in the EDTA control experiment, indicating its secretion is not influenced by iron depletion. Since this metabolite does not appear in the GNPS database, we used SIRIUS31 to gain more information on its structure. SIRIUS uses a combination of techniques, including isotope pattern analysis, molecular formula prediction and fragmentation tree computation, to identify the most likely structures for a given mass spectral feature. The proposed structure of this metabolite was methyl phenazine-1-carboxylate (MPCA, Figure 6B), a derivative of phenazine-1-carboxylic acid (PCA), with 91 % certainty (Figure S7). The validation of the MPCA structure was further confirmed by us through preparation of a standard (and comparison of NMR and UPLC-HRMS data), synthesized by esterification of commercially available PCA (Figures S8A–C), which provided level I structure identification. Phenazines belong to a family of molecules with a dibenzo-annulated pyrazine core and they differ in the position and types of functional groups. They are highly studied because of their roles in virulence and antimicrobial properties.32, 33 PCA is a precursor to other phenazine derivatives, such as 1-hydroxyphenazine, phenazine-1-carboxamide (PCN) and pyocyanin, a hallmark of Pseudomonas virulence.34, 35 Although some phenazines, such as PCA, are used by P. aeruginosa for iron acquisition under limiting conditions,36, 37 in this experiment PCA production remained equal throughout the varying concentration of PME (Figure S9A). In addition, there was no change in pyochelin secretion in the presence of PME (Figure S9A), while both were induced in the EDTA control experiment (Figure S9B), indicating PME does not cause iron starvation in the used concentrations and MPCA appears to be directly related to the presence of PME.
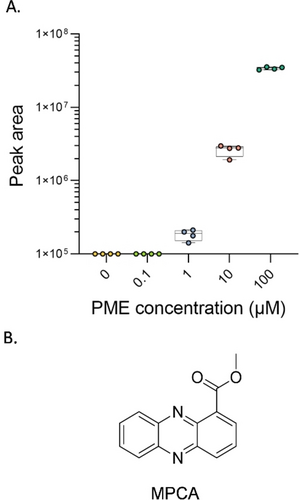
A) PME induces the production of a metabolite secreted by P. aeruginosa, identified as methyl phenazine-1-carboxylate (MPCA). The box plots indicate the median (line), the interquartile range (box), and the extreme values (whiskers) of the chromatogram peak intensity. B) The suggested and validated structure for the identified MPCA.
MPCA was previously found in soil samples containing Streptomyces canus,38 and showed antifungal activities, stronger than PCA in some cases.39, 40 It was also tested for antibacterial activity against MRSA but exhibited only mild inhibitory effects.41 Given that two enzymes are already known to convert PCA to different derivatives (phzM and phzS) in P. aeruginosa,34 it is reasonable to consider the possibility of the existence of additional, unknown enzymes that can convert PCA to other derivatives such as MPCA in response to the needs of the bacteria. Based on these initial findings, the influence of PME on phenazine production is very intriguing and could be related to the reported increased pathogenesis of this dual-species biofilm.5, 6 These results strengthen our hypothesis that PME is more than a by-product of P. aeruginosa—S. aureus encounters; the transformation of pyochelin to PME enables S. aureus to better compete for available iron, but it may also serve S. aureus to signal its presence to P. aeruginosa and coordinate their behavior; of course, further investigations are warranted to explore this enticing hypothesis.
Conclusion
P. aeruginosa and S. aureus co-infections are associated with persistent colonization of human airways and deleterious disease outcomes. However, little is known about the mechanisms that enable coexistence between these species in specific environments. Using an untargeted metabolomics approach, we discovered a particular change in the metabolic profile of the mutual biofilm. We found that PME, a derivative of pyochelin, appears with increasing concentrations upon increasing S. aureus inoculum concentrations at the start of formation of these biofilms. We demonstrated that S. aureus possesses an enzyme that converts pyochelin to PME, disabling its ability to chelate iron and deliver it to P. aeruginosa. While detailed mechanisms behind this transformation remain to be elucidated and are part of our ongoing investigations. To the best of our knowledge, our study and the report by Jenul et al.25 are the first reported examples of this type of interaction between P. aeruginosa and S. aureus. Since pyochelin is an inhibitor of S. aureus, its conversion to PME reveals a protection mechanism for S. aureus to allow its growth in the presence of P. aeruginosa and share public goods.
Besides protecting S. aureus, PME might be also used as a signaling molecule that could benefit P. aeruginosa; by detecting PME, P. aeruginosa may be able to sense the presence of S. aureus and alter its behavior accordingly by adjusting its virulence toward the potential competitor and either compete or allow cooperation, depending on its relative population density.
This study adds another piece to the complex puzzle of interactions between different microbes such as P. aeruginosa and S. aureus. We suspect that the conversion of pyochelin to PME plays an important role in guiding the make-up of mutual biofilms. Several important questions regarding the regulation of these processes remain open and these are the subject of further investigations.
Acknowledgments
This study was supported by a grant from the Israel Science Foundation (M.M.M., Grant #1485/20). We thank Prof. Ariel Kushmaro (Ben-Gurion University) for generously providing the wild-type MRSA strain. We also gratefully acknowledge Prof. Alexander Horswill (Univ. of Colorado, Anschutz School of Medicine) for helpful discussions.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.