Mechanochemical Approach for Air-Tolerant and Extremely Fast Lithium-Based Birch Reductions in Minutes
Dr. Yunpeng Gao
Division of Applied Chemistry, Graduate School of Engineering, Hokkaido University, 060-8628 Sapporo, Hokkaido, Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Koji Kubota
Division of Applied Chemistry, Graduate School of Engineering, Hokkaido University, 060-8628 Sapporo, Hokkaido, Japan
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University, 060-0021 Sapporo, Hokkaido, Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Hajime Ito
Division of Applied Chemistry, Graduate School of Engineering, Hokkaido University, 060-8628 Sapporo, Hokkaido, Japan
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University, 060-0021 Sapporo, Hokkaido, Japan
Search for more papers by this authorDr. Yunpeng Gao
Division of Applied Chemistry, Graduate School of Engineering, Hokkaido University, 060-8628 Sapporo, Hokkaido, Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Koji Kubota
Division of Applied Chemistry, Graduate School of Engineering, Hokkaido University, 060-8628 Sapporo, Hokkaido, Japan
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University, 060-0021 Sapporo, Hokkaido, Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Hajime Ito
Division of Applied Chemistry, Graduate School of Engineering, Hokkaido University, 060-8628 Sapporo, Hokkaido, Japan
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University, 060-0021 Sapporo, Hokkaido, Japan
Search for more papers by this authorGraphical Abstract
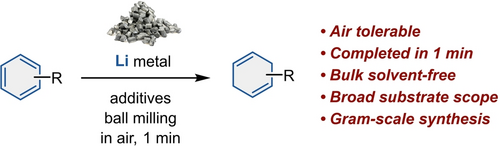
A mechanochemical Birch reduction is reported for the first time. The newly developed ball-milling method does not require an inert atmosphere or other special precautions. Notably, the reaction reached completion within one minute for most of the investigated substrates. The present study thus provides a novel, operationally simple, rapid, and scalable alternative to conventional solution-based Birch reduction.
Abstract
Birch reduction has been widely used in organic synthesis for over half a century as a powerful method to dearomatize arenes into 1,4-cyclohexadiene derivatives. However, the conventional Birch reduction reaction using liquid ammonia requires laborious procedures to ensure inert conditions and low temperatures. Although several ammonia-free modifications have been reported, the development of an operationally simple, efficient, and scalable protocol remains a challenge. Herein, we report an ammonia-free lithium-based Birch reduction in air without special operating conditions using a ball-milling technique. This method is characterized by its operational simplicity and an extremely short reaction time (within 1 min), probably owing to the in situ mechanical activation of lithium metal, broad substrate scope, and no requirement for dry bulk solvents. The potential of our flash Birch reaction is also demonstrated by the efficient reduction of bioactive target molecules and gram-scale synthesis.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202217723-sup-0001-misc_information.pdf11.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. J. Birch, J. Chem. Soc. 1944, 430–436;
- 1bA. L. Wilds, N. A. Nelson, J. Am. Chem. Soc. 1953, 75, 5360–5365.
- 2For a review of the application of Birch reduction in natural product synthesis, see: J. M. Hook, L. N. Mander, Nat. Prod. Rep. 1986, 3, 35–85.
- 3For a review of the mechanistic studies of Birch reduction, see: H. E. Zimmerman, Acc. Chem. Res. 2012, 45, 164–170.
- 4For selected examples of electrochemical Birch reduction, see:
- 4aB. K. Peters, K. X. Rodriguez, S. H. Reisberg, S. B. Beil, D. P. Hickey, Y. Kawamata, M. Collins, J. Starr, L. Chen, S. Udyavara, K. Klunder, T. J. Gorey, S. L. Anderson, M. Neurock, S. D. Minteer, P. S. Baran, Science 2019, 363, 838–845;
- 4bK. Hayashi, J. Griffin, K. C. Harper, Y. Kawamata, P. S. Baran, J. Am. Chem. Soc. 2022, 144, 5762–5768.
- 5For selected examples of photochemical Birch reduction, see:
- 5aJ. P. Cole, D.-F. Chen, M. Kudisch, R. M. Pearson, C.-H. Lim, G. M. Miyake, J. Am. Chem. Soc. 2020, 142, 13573–13581;
- 5bA. Chatterjee, B. König, Angew. Chem. Int. Ed. 2019, 58, 14289–14294;
- 5cK. Mizuno, H. Okamoto, C. Pac, H. Sakurai, J. Chem. Soc. Chem. Commun. 1975, 839–840;
- 5dY. Yoshimi, A. Ishise, H. Oda, Y. Moriguchi, H. Kanezaki, Y. Nakaya, K. Katsuno, T. Itou, S. Inagaki, T. Morita, M. Hatanaka, Tetrahedron Lett. 2008, 49, 3400–3404.
- 6For selected examples of ammonia-free Birch reduction using alkali metals, see:
- 6aT. J. Donohoe, D. House, J. Org. Chem. 2002, 67, 5015–5018;
- 6bJ. L. Dye, K. D. Cram, S. A. Urbin, M. Y. Redko, J. E. Jackson, M. Lefenfeld, J. Am. Chem. Soc. 2005, 127, 9338–9339;
- 6cP. Lei, Y. Ding, X. Zhang, A. Adijiang, H. Li, Y. Ling, J. An, Org. Lett. 2018, 20, 3439–3442;
- 6dS. Asako, I. Takahashi, T. Kurogi, Y. Murakami, L. Ilies, K. Takai, Chem. Lett. 2022, 51, 38–40.
- 7
- 7aR. A. Benkeser, R. E. Robinson, D. M. Sauve, O. H. Thomas, J. Am. Chem. Soc. 1955, 77, 3230–3233;
- 7bR. A. Benkeser, C. Arnold, R. F. Lambert, O. H. Thomas, J. Am. Chem. Soc. 1955, 77, 6042–6045.
- 8L. Reggel, R. A. Friedel, I. Wender, J. Org. Chem. 1957, 22, 891–894.
- 9M. E. Garst, L. J. Dolby, S. Esfandiari, N. A. Fedoruk, N. C. Chamberlain, A. A. Avey, J. Org. Chem. 2000, 65, 7098–7104.
- 10J. Burrows, S. Kamo, K. Koide, Science 2021, 374, 741–746.
- 11For selected reviews on organic synthesis using mechanochemistry, see:
- 11aS. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed, D. C. Waddell, Chem. Soc. Rev. 2012, 41, 413–447;
- 11bG.-W. Wang, Chem. Soc. Rev. 2013, 42, 7668–7700;
- 11cJ. G. Hernández, C. Bolm, J. Org. Chem. 2017, 82, 4007–4019;
- 11dD. Tan, T. Friščić, Eur. J. Org. Chem. 2018, 18–33;
- 11eJ. L. Howard, Q. Cao, D. L. Browne, Chem. Sci. 2018, 9, 3080–3094;
- 11fJ. Andersen, J. Mack, Green Chem. 2018, 20, 1435–1443;
- 11gD. Tan, F. García, Chem. Soc. Rev. 2019, 48, 2274–2292;
- 11hC. Bolm, J. G. Hernández, Angew. Chem. Int. Ed. 2019, 58, 3285–3299;
- 11iT. Friščić, C. Mottillo, H. M. Titi, Angew. Chem. Int. Ed. 2020, 59, 1018–1029;
- 11jK. Kubota, H. Ito, Trends Chem. 2020, 2, 1066–1081;
- 11kI. N. Egorov, S. Santra, D. S. Kopchuk, I. S. Kovalev, G. V. Zyryanov, A. Majee, B. C. Ranu, V. L. Rusinov, O. N. Chupakhin, Green Chem. 2020, 22, 302–315;
- 11lA. Porcheddu, E. Colacino, L. De Luca, F. Delogu, ACS Catal. 2020, 10, 8344–8394;
- 11mK. J. Ardila-Fierro, J. G. Hernández, ChemSusChem 2021, 14, 2145–2162;
- 11nS. Hwang, S. Grätz, L. Borchardt, Chem. Commun. 2022, 58, 1661–1671;
- 11oR. R. A. Bolt, J. A. Leitch, A. C. Jones, W. I. Nicholson, D. L. Browne, Chem. Soc. Rev. 2022, 51, 4243–4260;
- 11pM. T. J. Williams, L. C. Morrill, D. L. Browne, ChemSusChem 2022, 15, e202102157;
- 11qF. Cuccu, L. De Luca, F. Delogu, E. Colacino, N. Solin, R. Mocci, A. Porcheddu, ChemSusChem 2022, 15, e202200362.
- 12
- 12aT. Seo, K. Kubota, H. Ito, J. Am. Chem. Soc. 2020, 142, 9884–9889;
- 12bL. Pan, L. Zheng, Y. Chen, Z. Ke, Y.-Y. Yeung, Angew. Chem. Int. Ed. 2022, 61, e202207926;
- 12cK. J. Ardila-Fierro, M. Rubčić, J. G. Hernández, Chem. Eur. J. 2022, 28, e202200737.
- 13
- 13aT. Seo, N. Toyoshima, K. Kubota, H. Ito, J. Am. Chem. Soc. 2021, 143, 6165–6175;
- 13bY. Gao, C. Feng, T. Seo, K. Kubota, H. Ito, Chem. Sci. 2022, 13, 430–438;
- 13cG.-W. Wang, K. Komatsu, Y. Murata, M. Shiro, Nature 1997, 387, 583–586.
- 14
- 14aK. Kubota, R. Takahashi, H. Ito, Chem. Sci. 2019, 10, 5837–5842;
- 14bR. Takahashi, K. Kubota, H. Ito, Chem. Commun. 2020, 56, 407–410;
- 14cF. J. L. Ingner, Z. X. Giustra, S. Novosedlik, A. Orthaber, P. J. Gates, C. Dyrager, L. T. Pilarski, Green Chem. 2020, 22, 5648–5655;
- 14dG. Pisanò, C. S. J. Cazin, ACS Sustainable Chem. Eng. 2021, 9, 9625–9631;
- 14eS. Ni, M. Hribersek, S. K. Baddigam, F. J. L. Ingner, A. Orthaber, P. J. Gates, L. T. Pilarski, Angew. Chem. Int. Ed. 2021, 60, 6660–6666.
- 15For a tutorial review on the mechanical activation of zero-valent metals, see: A. C. Jones, J. A. Leitch, S. E. Raby-Buck, D. L. Browne, Nat. Synth. 2022, 1, 763–775.
- 16
- 16aQ. Cao, J. L. Howard, E. Wheatley, D. L. Browne, Angew. Chem. Int. Ed. 2018, 57, 11339–11343;
- 16bQ. Cao, R. T. Stark, I. A. Fallis, D. L. Browne, ChemSusChem 2019, 12, 2554–2557;
- 16cJ. Yin, R. T. Stark, I. A. Fallis, D. L. Browne, J. Org. Chem. 2020, 85, 2347–2354;
- 16dA. C. Jones, W. I. Nicholson, J. A. Leitch, D. L. Browne, Org. Lett. 2021, 23, 6337–6341.
- 17
- 17aR. Takahashi, P. Gao, K. Kubota, H. Ito, Chem. Sci. 2023, 14, 499–505;
- 17bW. Nicholson, J. Howard, G. Magri, A. Seastram, A. Khan, R. R. A. Bolt, L. Morrill, E. Richards, D. L. Browne, Angew. Chem. Int. Ed. 2021, 60, 23128–23133.
- 18S. Wu, W. Shi, G. Zou, New J. Chem. 2021, 45, 11269–11274.
- 19
- 19aR. Takahashi, A. Hu, P. Gao, Y. Gao, Y. Pang, T. Seo, J. Jiang, S. Maeda, H. Takaya, K. Kubota, H. Ito, Nat. Commun. 2021, 12, 6691;
- 19bC. Wu, T. Ying, X. Yang, W. Su, A. V. Dushkin, J. Yu, Org. Lett. 2021, 23, 6423–6428.
- 20
- 20aV. S. Pfennig, R. C. Villella, J. Nikodemus, C. Bolm, Angew. Chem. Int. Ed. 2022, 61, e202116514;
- 20bH. Chen, J. Fan, Y. Fu, C.-L. Do-Thanh, X. Suo, T. Wang, I. Popovs, D. Jiang, Y. Yuan, Z. Yang, S. Dai, Adv. Mater. 2021, 33, 2008685;
- 20cI. R. Speight, T. P. Hanusa, Molecules 2020, 25, 570.
- 21P. Gao, J. Jiang, S. Maeda, K. Kubota, H. Ito, Angew. Chem. Int. Ed. 2022, 61, e202207118.
- 22T. J. Donohoe, R. E. Thomas, Nat. Protoc. 2007, 2, 1888–1895.
- 23N. Davison, J. A. Quirk, F. Tuna, D. Collison, C. L. McMullin, H. Michaels, G. H. Morritt, P. G. Waddell, J. A. Gould, M. Freitag, J. A. Dawson, E. Lu, Chem 2023, 9, 576–591.
- 24M. A. González, D. Pérez-Guaita, J. Correa-Royero, B. Zapata, L. Agudelo, A. Mesa-Arango, L. Betancur-Galvis, Eur. J. Med. Chem. 2010, 45, 811–816.
- 25Although we did not encounter any hazards in the gram-scale mechanochemical Birch reduction in air, anyone who wants to scale up should pay attention to the safety issues. On one hand, organolithiums and lithium metal are commonly considered to be pyrophoric. On the other hand, Birch reduction is an exothermic reaction that might release flammable vapors. Risk management must be done to avoid fire and explosion hazards before any attempts to scale up this reaction in air.