Tailoring Active Cu2O/Copper Interface Sites for N-Formylation of Aliphatic Primary Amines with CO2/H2
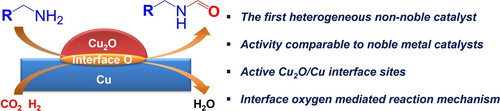
Graphical Abstract
Abstract
Heterogeneously catalyzed N-formylation of amines to formamide with CO2/H2 is highly attractive for the valorization of CO2. However, the relationship of the catalytic performance with the catalyst structure is still elusive. Herein, mixed valence catalysts containing Cu2O/Cu interface sites were constructed for this transformation. Both aliphatic primary and secondary amines with diverse structures were efficiently converted into the desired formamides with good to excellent yields. Combined ex and in situ catalyst characterization revealed that the presence of Cu2O/Cu interface sites was vital for the excellent catalytic activity. Density functional theory (DFT) calculations demonstrated that better catalytic activity of Cu2O/Cu(111) than Cu(111) is attributed to the assistance of oxygen at the Cu2O/Cu interface (Ointer) in formation of Ointer-H moieties, which not only reduce the apparent barrier of HCOOH formation but also benefit the desorption of the desired N-formylated amine, leading to high activity and selectivity.
Introduction
The replacement of fossil carbon resources by renewable ones is a major challenge to develop sustainable chemistry. CO2 is a non-toxic, cheap, abundant and sustainable C1 resource, and its transformation into valuable chemicals and fuels has attracted wide attention.1 Formamides are important intermediates and solvents in industry and their synthesis has emerged as an intensely studied topic in chemistry.2 To replace highly toxic CO used for industrial production of formamides,3 various safer carbonyl source reagents such as formic acid or formate,4 formaldehyde,5 methanol,6 ethers,7 N,N-dimethylformamide8 and so on have been developed.2a, 9 N-formylation of amines with CO2/H2, using preferentially homogeneous noble metal catalysts is one of the main methods for synthesis of formamides.2c, 10 However, from the view of industrial production, homogeneous processes are less desired,10 due to inherent difficulty in product separation and catalyst recycling. Although some heterogeneous catalysts have been reported, they suffer from the use of noble metals such as Pd,11 Ru12 and Au,13 or alkaline additives,11b, 11d, 11e, 11h high temperature (200 °C),13b and/or high pressure (12 MPa).14 Moreover they are not active for converting aliphatic primary amine substrates.14, 15 To the best of our knowledge, active heterogeneous catalysts based on non-noble metals have not yet been reported for N-formylation of aliphatic primary amines with CO2/H2.
In the N-formylation of amines with CO2/H2, HCOOH has been reported to be the key intermediate in both HCOOH and HCOOMe pathways.11f The further hydrogenation of formamides to the corresponding methylamines is the main side reaction.16, 17 Therefore, to obtain the desired formamide, it is vital to control the selective hydrogenation of CO2 to HCOOH and prevent the over-reduction of formamides to methylamines. It has been reported that HCOO* is a key intermediate to synthesize HCOOH.18 Thus, it should be feasible to construct a bifunctional interface, which is both active for the selective hydrogenation of CO2 to HCOOH and favorable to the desorption of the formamide, for the challenging transformation of aliphatic primary amines into the corresponding formamides with CO2/H2. Recently, it has been reported that heterostructure CuOx/Cu exhibited superior performance in CO2 hydrogenation to methanol compared to single CuOx and metallic Cu sites.19
Against this background, here we for the first time achieved the challenging N-formylation of aliphatic primary amines with CO2/H2 by constructing an active Cu2O/Cu phase that not only efficiently accelerates the formation of HCOOH intermediate, but also prevent the further reduction of the formamide products. When dispersing on an Al2O3 matrix, more active Cu2O/Cu phase were obtained and the agglomeration of the active sites was efficiently prevented, enabling the recycling of the active catalyst several times without obvious activity loss. We found that the Ointer at the Cu2O/Cu interface played the crucial factor via the creation of Ointer-H moieties during CO2 hydrogenation, favoring the selective formation of HCOO* species.20 Moreover, the electron-rich Cu0 center is favorable to the desorption of the N-formylated molecules, thereby inhibiting the over-reduction to N-methylated amines and promoting the selectivity.
Results and Discussion
The catalytic performance for N-formylation of octylamine on catalysts pre-reduced at different temperatures and times is shown in Figure 1. Only less than 10 % of formamide was obtained over fresh unsupported CuOx without any reductive pretreatment (CuOx-NR). This suggests that CuII, being the main component in this catalyst, is less active for this reaction. This is also true for sample CuOx-150-1h, indicating that CuOx can hardly be reduced at 150 °C during 1 h. Upon raising the reduction temperature from 210 to 450 °C, the formaldehyde yields show a volcano-type behavior with a maximum at 250 °C. This suggests, in agreement with characterization data presented below, that deep reduction of CuII to Cu0 occurring at higher temperature lowered the catalytic activity towards formamides. The same effect was caused by prolonging the reduction time, as evident from a comparison of samples CuOx-250-1h and CuOx-250-3h (Figure 1a). As shown by pseudo in situ X-ray photoelectron spectroscopy (XPS) data below, the latter sample contained mainly metallic Cu in addition to a small amount of CuI.
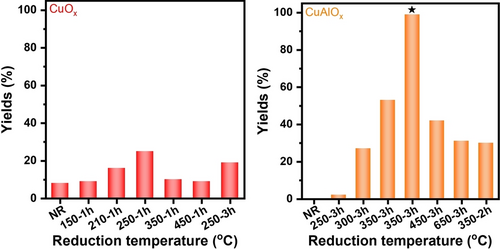
Yields of formamide products as a function of catalyst pre-reduction temperature and time for a) CuOx, (reaction conditions: 1 mmol octylamine, 50 mg catalyst, 3 MPa H2, 1 MPa CO2, 3 mL MeOH, oven temperature 150 °C, 3 h) and b) CuAlOx (reaction conditions: 1 mmol octylamine, 100 mg catalyst, 3 MPa H2, 1 MPa CO2, 3 mL MeOH, oven temperature 130 °C, 12 h; * 150 °C, 24 h).
Furthermore, the recyclability of sample CuOx-250-1h was investigated. While a formamide yield of 25 % was quickly reached within 3 h over the fresh CuOx-250-1h catalyst, but only 53 % yield was obtained after 24 h. This deactivation may be due to agglomeration of the active Cu sites to larger metallic Cu particles during the reaction (Figure S1), probably promoted by reduction of CuOx under reaction conditions.
To suppress agglomeration of the active species during reaction, we deposited the CuOx phase on an alumina support, which is well known for stabilizing Cu sites21 and has been widely used in reductive amination of CO2.11j, 15a, 22 The CuAlOx catalyst with a nominal Cu content of 7.5 wt % was prepared by the same method as the CuOx catalyst and reduced at different temperature between 250 to 650 °C for 3 h. Due to the high catalytic performance of CuAlOx catalysts, their catalytic performance was tested at a lower reaction temperature than that of CuOx catalysts (Figure 1b). No formamide was formed on the non-reduced catalyst (CuAlOx-NR) in which CuII is dominating. Only a trace amount of formamide was observed on sample CuAlOx-250-3h. This can be attributed to the strong stabilization effect of Al2O3 on copper oxide.21 Raising the reduction temperature from 300 to 650 °C revealed a tendency in product yields similar to that of the unsupported CuOx catalyst and the maximum yield was obtained at 350 °C. A shorter reduction time led to a lower product yield, as seen from a comparison of CuAlOx samples reduced for 3 h and 2 h at 350 °C (Figure 1b). A CO2/H2 pressure ratio of 1/3 turned out to be optimal at a total pressure of 4 MPa (Table S1, entries 1–3). Prolonging the reaction time did not significantly improve the product yield (Table S1, entries 1 and 4). An excellent yield of 99 % was obtained when increasing the reaction temperature to 150 °C (Figure 1b). Reducing the Cu loading from 7.5 wt % to 1.9 wt % led to a continuous decrease in product yields (Figure 1b and Table S1, entries 6 and 7). No formamide products were observed after removing CO2 from the reaction system, meaning that methanol can't react with amines directly to form formamide product (Table S1, entry 7). In addition, the desired product was obtained using toluene and 1-octane as solvents, indicating CO2 was the real carbonyl source (Table S1, entries 8 and 9).
With the most active catalyst CuAlOx-350-3h, the substrate scope was further investigated (Table 1). Linear- and branched-chain aliphatic primary amines with C4–C16 number of carbon atoms could be converted with good to excellent formamide yields (81–96 %). 96 % and 82 % product yields were respectively obtained with cyclic aliphatic primary amines, cyclopentamine, and phenyl-substituted aliphatic primary amine, 3-phenylpropan-1-amine. Benzyl amine and its derivatives with a series of different groups such as -Me, -CH(Me)2, -OMe, and -F can also provide the corresponding formamide products in 77–96 % yields. Moreover, the number and position of substituted groups have little effect on the activity and no obvious electronic and spatial resistance effect were observed. This system is also active for cyclohexylmethanamine and cyclopropylmethanamine and 75 % and 98 % product yields were obtained, respectively. Besides, secondary amines with different structures could be converted effectively. 84–99 % product yields have been obtained from cyclic secondary amines such as piperidine, 4-methylpiperidine, 3,5-dimethylpiperidine, morpholine and pyrrolidine as substrates. In comparison to those, dimethylamine and N-benzylethanamine showed slightly lower reactivity, providing moderate to good product yields.
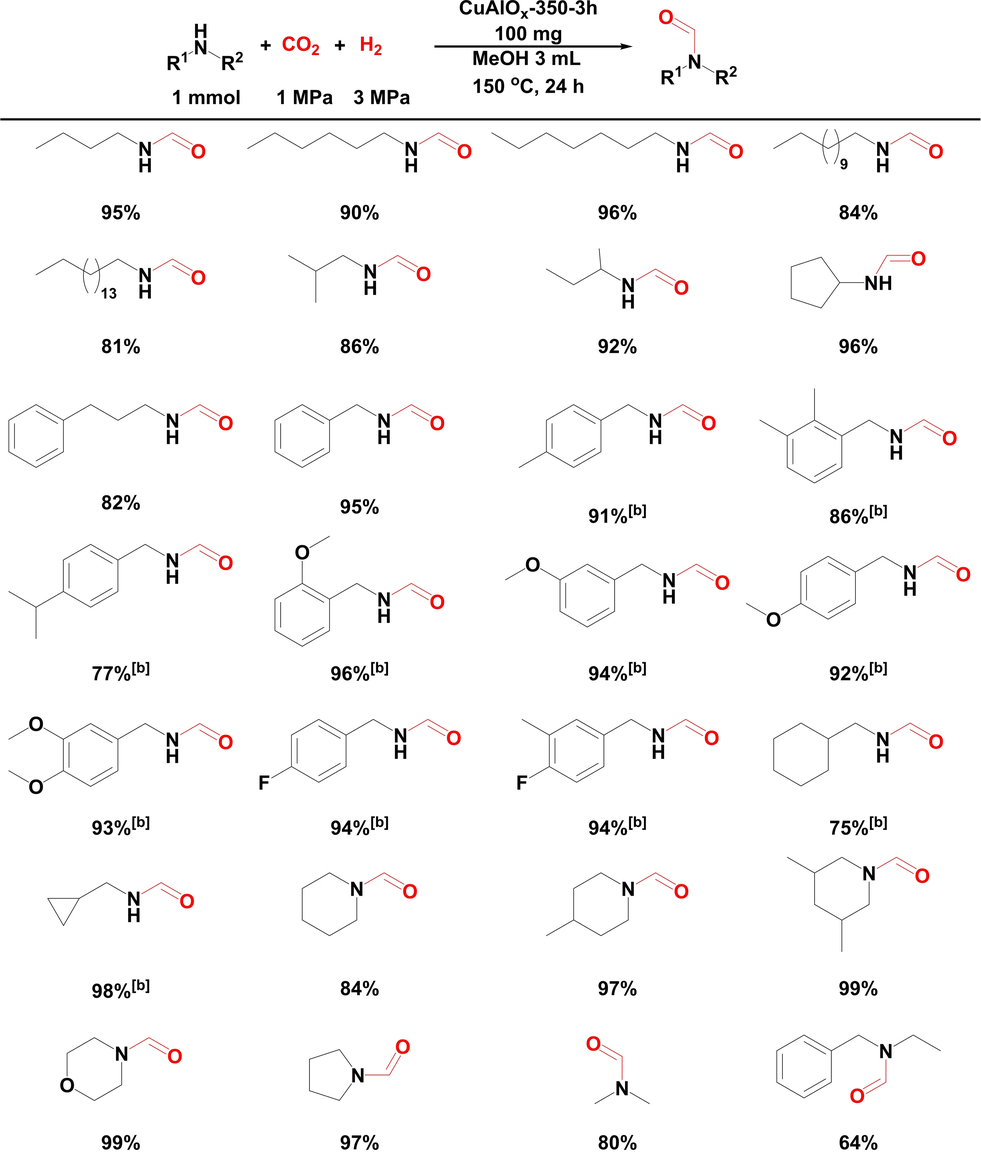
- [a] Yields were determined by GC-FID with biphenyl as the external standard. [b] Yields were determined by 1H NMR with triphenylmethane as the internal standard.
Inspired by the good substrate scope of this catalytic system in the saturated amines, the selective N-formylation of amines containing unsaturated groups with CO2 and H2, which is challenging because the efficient catalysts for the N-formylation are usually very active for hydrogenation of the unsaturated groups, is explored. Gladly, the substrate piperidine-4-carboxamide was converted to the desired formamides in 92 % yields, while the unsaturated groups (C≡N bond) remained (Scheme 1). Moreover, this system is also chemoselective for the molecules containing both the hydroxyl and amino groups, and only N-formylated product was formed under the optimized reaction conditions. For example, 5-aminopentan-1-ol delivered the corresponding formamide product in 96 % yields (Scheme 1).
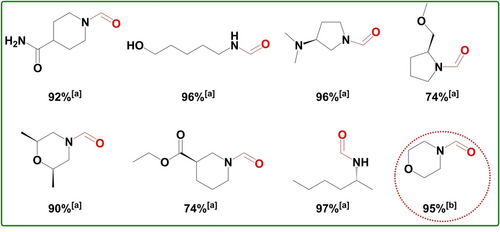
Reductive amination of CO2 with amines containing unsaturated groups, hydroxyl groups and chiral centers as well as gram-scale reaction. [a] Yields were determined by 1H NMR with triphenylmethane as the internal standard. [b] Reaction conditions: 15 mmol morpholine, 3 MPa H2, 1 MPa CO2, 1.5 g CuAlOx-350-3h, 45 mL MeOH, 150 °C, 24 h. Yields were determined by GC-FID with biphenyl as the external standard.
It was notable that this methodology can be also extended to the chiral amines, which are widely applied as the synthetic intermediates for the synthesis of the drug and bioactive molecules.23 74–97 % yields of chiral formamides were gained when chiral amines such as (R)-N,N-dimethylpyrrolidin-3-amine, (R)-2-(methoxymethyl)pyrrolidine, (2R,6S)-2,6-dimethylmorpholine, ethyl (S)-piperidine-3-carboxylate and (R)-hexan-2-amine were used as substrates (Scheme 1). To demonstrate the utility of this methodology, the active catalyst CuAlOx-350-3h was used for the gram-scale synthesis of formamide. The N-formylation of morpholine was taken as an example and the corresponding morpholine-4-carbaldehyde (about 1.7 g) was obtained in 95 % yield (Scheme 1).
Finally, the recyclability of the active catalyst CuAlOx-350-3h was investigated. Compared to the most active unsupported CuOx catalyst, the introduction of aluminum oxide strongly raised the stability and this catalyst could be consecutively used for 6 times without obvious activity loss (Figure S2). Inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis of the CuAlOx-350-3h catalyst in before and after use revealed that the Cu content slightly decreased from 6.70 wt % in the fresh catalyst to 6.56 wt % after the 6th run, suggesting that the catalyst is stable. To demonstrate the heterogeneous nature of the active catalyst and exclude the contribution of leached Cu, the catalyst was removed by hot filtration after 6 h when 35 % product yield was measured. During further reaction of the clear solution for another 18 h after catalyst removal, no further product was formed, which clearly indicates that the solid Cu supported on alumina is the active species.
To reasonably assess our catalytic system, the catalytic performance of the most active CuAlOx-350-3h catalyst in our system for N-formylation of primary and secondary amines was respectively compared with that of the reported heterogeneous catalysts in the literatures. Considering the wide use of C6–C8 aliphatic primary amines and morpholine as typical starting materials, their N-formylation were used as the model reaction to evaluate the catalytic performance of our catalyst and the reported catalysts (Table S3). Clearly, the catalytic performance of our catalyst is comparable to the reported noble metal catalysts and even much better than most of them, especially in the case of aliphatic primary amines.
To unravel a correlation between structure and activity, different ex and in situ characterization techniques have been applied such as in situ X-ray diffraction (XRD), XPS, high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) and electron energy loss spectroscopy (EELS). N2-adsorption and desorption analysis showed that the calcined unsupported CuOx catalyst had a very small specific surface area (14.6 m2 g−1) and pore volume (0.10 cm3 g−1), which even decreased after reductive pretreatment (Table S2). In contrast, these values were much higher for calcined CuAlOx and were retained widely after pre-reduction for 3 h at 350 °C (142.1 m2 g−1 and 0.4878 cm3 g−1, Table S2).
In situ XRD powder patters of CuOx catalysts reduced at different temperature (Figure 2a and b) showed only CuO diffraction peaks (JCPDS, Card No. 80-1916)24 at 25 and 190 °C. New peaks from Cu2O (111 and 200) (JCPDS, Card No. 65-3288)25 appeared at 210 °C (Figure 2b). At higher reduction temperature (220–350 °C), the peak intensity of metallic Cu (JCPDS, Card No. 04-0836)26 and Cu2O gradually increased while that of CuO decreased. CuO was totally reduced to Cu and Cu2O at 230 °C. Despite the fact that the Cu2O peaks disappeared at 250 °C, this phase may still be present in amorphous or nanocrystalline form, as suggested by pseudo in situ XPS presented below. Thus, the co-existence of both Cu and Cu2O in the CuOx-250-1h catalyst is possible. The XRD powder patterns of the most active supported catalyst CuAlOx-350-3h (Figure 2c) show only weak peaks of metallic Cu(111), Cu(200), Cu(220) and Cu2O(111),25 indicating the co-existence of both Cu and Cu2O which did not change after four consecutive runs in the catalytic reaction for 24 h at 150 °C.
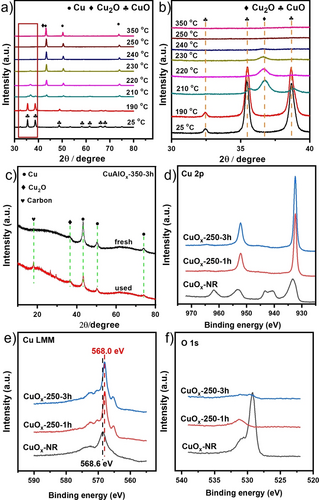
a) In situ XRD powder patterns of CuOx reduced at different temperatures and b) the corresponding enlarged of 30–40 degree. c) Ex situ XRD patterns of fresh and used CuAlOx-350–3h. Pseudo in situ XPS spectra of CuOx: d) Cu 2p, e) Cu LMM Auger and f) O 1s peaks.
Considering the sensitivity of Cu0/I species to air,27 pseudo in situ XPS measurement were performed to reveal the real active surface of the CuOx-250-1h catalyst (Figure 2d–f). The non-reduced CuOx-NR catalyst shows characteristic CuO peaks with a broad satellite peak centered at around 944 eV and a typical Cu 2p3/2 binding energy of 934.5 eV from CuII species (Figure 2d).28 This was also in agreement with the corresponding Cu LMM Auger peak at 568.6 eV which showed the typical shape observed for CuO (Figure 2e).29 After treatment with H2 at 250 °C for 1 h, the satellite peak of CuII disappeared while the Cu LMM peak shifted to 568.0 eV and changed its shape becoming similar to that of Cu0,29 indicating that CuII species in the CuOx-NR catalyst were reduced, most probably mainly to Cu0. This was also in agreement with EELS results discussed below (Figure 3). However, since still an O 1s peak was observed after reductive treatment (Figure 2f), the presence of some residual oxidic copper cannot be excluded in the CuOx-250-1h catalyst. Increasing the reduction time at 250 °C to 2 h enhanced the changes observed after 1 h even more.
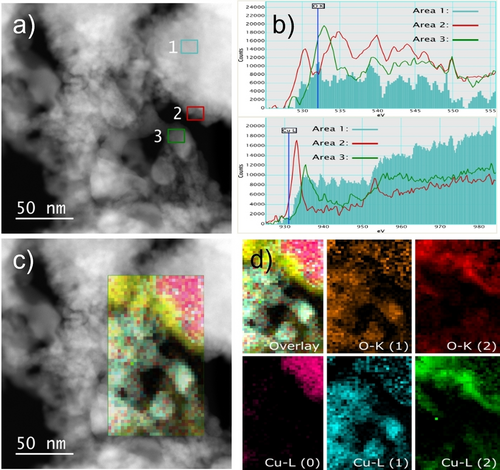
a) ADF-STEM image with marked areas corresponding to the EELS spectra recorded at the b) O-K (top) and Cu-L (bottom) edge of CuOx-250-1h with Cu0 in area 1, CuII in area 2 and CuI in area 3; c) ADF-STEM image overlayed with mixed color map, d) STEM-EELS maps showing the distribution of different types of oxygen and different oxidation states of Cu with the numbers in parenthesis designating the oxidation state of Cu.
Cu2p XPS spectra of CuAlOx reduced at different temperatures were shown in Figure S3. The strong satellite peak of CuII between 940–947 eV was observed in the catalyst CuAlOx-250-3h, suggesting the presence of CuII species. Fitting of the Cu 2p3/2 peak revealed another contribution around 932.4 eV that could be assigned to CuI/0.29a With increasing the reduction temperature, the typical satellite peak of CuII gradually weakened and disappeared after treatment at 650 °C for 3 h, suggesting that all CuII species were reduced to CuI/0. Furthermore, Cu LMM Auger spectra showed two peaks around 915 and 916.8 eV in the catalyst CuAlOx-250-3h, which can be assigned to CuII[30] and CuI,31 respectively, i.e., there was no CuI/Cu0 interface sites, probably reasoning for its poor activity. A new peak appeared at around 918.0 eV in the case of CuAlOx-300-3h, CuAlOx-350-3h, CuAlOx-450-3h and CuAlOx-650-3h, which can be attributed to the formation of Cu0.32 By fitting the Auger spectra (Figure S3), it can be calculated that the CuI/Cu0 ratios were 3.24, 1.60, 0.88 and 0.62 for the catalysts CuAlOx-300–3h, CuAlOx-350-3h, CuAlOx-450-3h and CuAlOx-650-3h, respectively. Plotting the product yields with the CuI/Cu0 ratio (Figure S3), the optimum CuI/Cu0 ratio is about 1.60 for these samples, and both increasing and decreasing this ratio lowered the product yield.
HAADF-STEM images of CuOx-250-1h showed rather large Cu0 particles (Figure 3a). EELS near edge structure confirmed the co-existence of Cu0, CuI and CuII (Figure 3b).33 STEM-EELS maps (Figure 3c and d) showed that the Cu0 particles were surrounded by an oxide layer where both CuI and CuII present different types of morphology (Figure 3d). Cu0 was more compact with a rather smooth surface, while CuI and CuII seem more akin a cloudy type with a rough surface in close contact with the Cu0 particle. Considering that pseudo in situ XPS results after reduction showed no evidence for CuII, it was probable that CuII found in the HAADF-STEM pictures of the pre-reduced catalyst stemmed from oxidation of Cu0/I by ambient air.
HAADF-STEM image of catalyst CuAlOx-350-3h showed a few Cu crystallites with three different lattice parameters, all corresponding roughly to metallic Cu within the margin of error (Figure S4a), although other particles seemed to be of oxide character. STEM-EDS data showed that the Cu containing particles were highly dispersed on the surface of Al2O3 (Figure S4b and c). Furthermore, HR-TEM image revealed an existence of Cu2O(111) with a crystal facet distance of 0.245 nm, which was adjacent to some Cu(111) crystal facets with a distance of 0.210 nm (Figure S4d).34 In connection with XRD and XPS results (Figure 2c and S3), it can be concluded that the CuAlOx-350-3h catalyst contained Cu2O/Cu interface sites, just like CuOx-250-1h.
Combination of recycling experimental results and the characterizations of ICP-AES, XRD and XPS of the used CuAlOx-350-3h catalyst revealed that the active Cu2O/Cu sites is stable during the catalytic reaction.
To reveal the effect of Al on the surface basicity of catalysts, the CO2-TPD patterns of CuOx and CuAlOx were recorded in the range of 30–600 °C (Figure S5). For CuOx, two broad peaks of CO2 desorption were observed in the range of 50–250 °C and 350–550 °C, suggesting that CO2 can be adsorbed on the surface of CuOx catalyst in two different ways. Compared to CuOx, CuAlOx showed similar but much stronger CO2 desorption peaks, indicating that the addition of Al greatly increased the adsorption of CO2. That may be one of the reasons for the better catalytic activity of CuAlOx than CuOx.
To understand the catalytic activity of Cu2O/Cu sites on the N-formylation of aliphatic amines with CO2 and the effects of oxygen at the Cu2O/Cu interface, DFT calculations were carried out. Based on the characterization results vide supra and the reported references,35 DFT calculations were performed comparatively on a pure Cu(111) surface and a Cu(111) surface modified with a Cu2O cluster (Cu2O/Cu(111), Figure S6). Considering the CO2 hydrogenation to formic acid acts as the rate-determining step, two pathways were calculated: 1) CO2+2 H→HCOO+H→HCOOH and 2) CO2+2 H→COOH+H→HCOOH. The optimal potential energy surfaces (PESs) are shown in Figure 4. On the pure Cu(111) surface (Figure 4a), starting from co-adsorbed CO2 and hydrogen (Figure S7), either a HCOO or a COOH intermediate can be formed via hydrogenation at the carbon or oxygen atom. Since formation of COOH had a high barrier and strong endothermic (Ea=1.78 eV, Er=0.55 eV36) this pathway had been disregarded and formation of a HCOO intermediate was favored with a barrier and reaction energy of 1.03 and −0.17 eV, respectively (Reaction 1, Figure S7). Subsequently, HCOO can be further hydrogenated to HCOOH or H2COO, whereby formation of HCOOH was more preferred thermodynamically (0.47 eV, Figure S8) and the barrier was 1.04 eV (Reaction 2, Figure S7). According to Figure 4a, the apparent barrier and reaction energy for transforming CO2 to HCOOH was 1.03 and 0.30 eV on Cu(111).
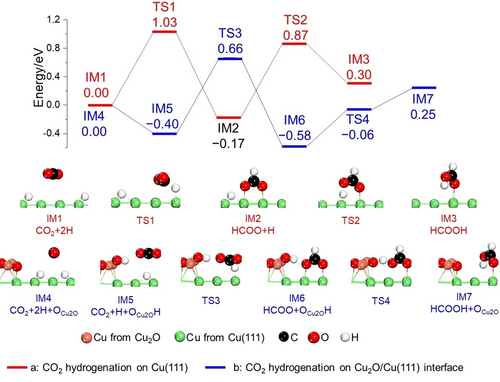
Energy profiles of CO2 hydrogenation to formic acid on a pure Cu(111) surface (red line, a) and on Cu2O/Cu(111) (blue line, b).
Although CO2 hydrogenation on a Cu2O(111) surface was more difficult than on Cu(111)35b and CO2 was weakly adsorbed on the catalyst surface (Figure S9), the CO2 hydrogenation was successfully proceeded due to the oxygen at Cu2O/Cu(111) surface and preferably initiated by the adsorption of the CO2+H2 at Cu(111) (Figure 4b). After the H2 activation at Cu(111) with the formation of two Cu−H species, one H specie can diffuse from the threefold coordinated site on the Cu(111) surface (0.00 eV) to the Ointer at the Cu2O/Cu interface, forming an Ointer-H moiety (−0.40 eV, Figure S10 top left, Figure 4b). Similar with that on the pure Cu(111) surface (Figure S7 and S8), the HCOO intermediate was more favorably generated by reacting Cu−H with C site of CO2 with the barrier and reaction energy of 1.06 eV and −0.18 eV (Reaction 3, Figure S10). Afterwards, the activated H on Ointer-H moiety was transferred to HCOO, producing the desired HCOOH at Cu2O/Cu(111) (Figure S11–S12) with barrier and reaction energy of 0.52 and 0.83 eV (Reaction 4, Figure S11). Since the reaction energy was higher than the barrier, formic acid formation was regarded as a barrierless reaction at Cu2O/Cu(111). Based on the DFT calculations vide supra, it was clearly revealed that the apparent barrier and reaction energy for CO2 hydrogenation to HCOOH was much lower on the Cu2O/Cu(111) interface (0.66 and 0.25 eV) than that on the pure Cu(111) surface (1.03 and 0.30 eV). Therefore, the presence of the Ointer not only reduced the apparent barrier of HCOOH formation but also changed the reaction of HCOO to HCOOH into a barrierless process. All these results strongly supported the idea of designing a heterostructured Cu2O/Cu catalyst.
Furthermore, the origin of the high selectivity of N-formylation of the amine on Cu2O/Cu interface was also investigated. The charge density difference (CDD) analysis was conducted to estimate the charge alteration of the Cu site during the formation of the Ointer-H species, and the results was displayed in Figure 5a–d and Supporting Information Figure S13, where the yellow and blue areas represented the higher and lower electron densities, respectively. Compared with the bare Cu2O/Cu model, the electron was transferred to Cu during the protonation of Cu2O/Cu with the increase of CDD. Therefore, the desorption properties of DMF were conducted on Cu2O/Cu and protonated Cu2O/Cu. Clearly, the distance between the oxygen of DMF and Cu site was elongated from 2.366 Å to 2.406 Å, indicating the easier desorption of DMF on protonated Cu2O/Cu interface (Figure 5c and d). Moreover, the desorption energy (Edes) of DMF on the Cu2O/Cu interfaces was 0.33 eV. However, the Edes of DMF over Cu2O(H)/Cu interface was decreased to 0.27 eV, suggesting that Cu2O/Cu interface with one OH served as more energetic sites for DMF adsorption (Figure 5e). This desorption effect modified by the protonated Cu2O/Cu interface makes the adsorption of N-formylated product harder and eventually contributed to their accelerated surface desorption and enhanced selectivity.
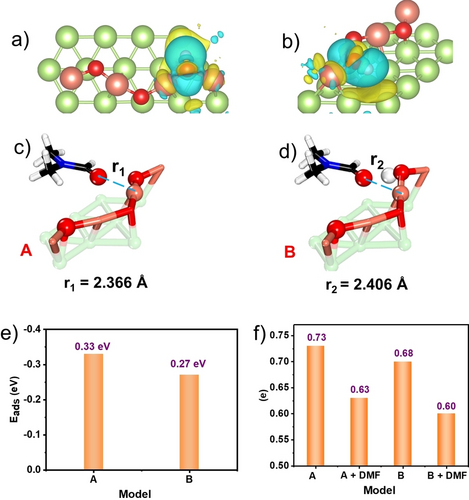
Top view (a) and side view (b) of charge density difference of model B. Yellow zone indicates electron enrichment, blue zone indicates electron deficiency; The adsorption models of DMF on Cu2O/Cu(111) interface (A) and Cu2O/Cu(111) after protonation (B), r1, r2 displayed the distances between O and Cu site (c, d), longer distance means easy desorption; (e) variation of the adsorption energies of DMF on model A and B with the absolute value, which is regarded as desorption energy, in red; (f) the Bader charges of Cu sites before and after DMF desorption (in units of e, less positive, higher electron density).
Bader charge analysis also approved that the Cu2O/Cu interfaces with OH site displayed the highest desorption energy. As shown in Figure 5f, the Bader charge analysis of bare Cu2O/Cu and Cu2OH/Cu with OH were 0.73 and 0.68, signifying the electron transfer from the O to Cu after the protonation of Cu2O/Cu during the reaction. After the adsorption of DMF, the net charge on Cu2O/Cu and Cu2OH/Cu was 0.63 and 0.60 respectively, indicating less charge transfer to the DMF molecules occurred at Cu2OH/Cu site. These results demonstrated that Cu site on Cu2OH/Cu was more electron-sufficient compared with that on Cu2O/Cu, favoring the desorption of this DMF molecules.37
To investigate the reaction intermediate, the mixtures of the model reaction after reaction for 12 h were monitored by 1H NMR and typical signals of HCOOMe were observed at 8.09 and 3.66 ppm (Figure S14). As the HCOOH had been confirmed to be the intermediate during the CO2 hydrogenation, both HCOOH and HCOOMe pathways were possible for the production of the N-formylated amines.11f Moreover, the reaction of HCOOH and CH3OH can quickly finished within 0.5 h (Scheme 2), indicating the formed HCOOH could be fast consumed and converted into HCOOMe quantitatively. In addition, the yield of N-formylated amine was quantitively obtained in the presence of HCOOMe while only 67 % yield was achieved using HCOOH as the reagent. Combined experimental results disclosed that the HCOOMe pathway displayed the major possibility for the formylation of amine using CO2 and H2.
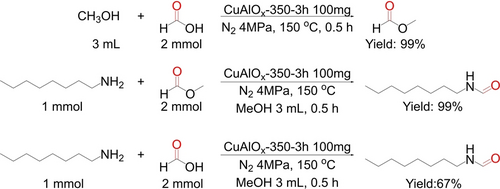
Control experiments on the formation of HCOOMe and N-formylated amine.
Based on the DFT calculations and experimental results, we believed that there are possibly three steps occurred during the N-formylation of amines with H2 and CO2 at Cu2O/Cu(111) interface (Scheme 3). Initially, facilitating by the Ointer at Cu2O/Cu(111), H2 was adsorbed and activated with the formation of Cu−H and Cu2OH which reacted with CO2 to produce HCOOH. Subsequently, the HCOOH was quickly converted to HCOOMe in methanol solution. Finally, the generated HCOOMe directly reacting with amine under heating conditions to produce the desired N-formylated amine.
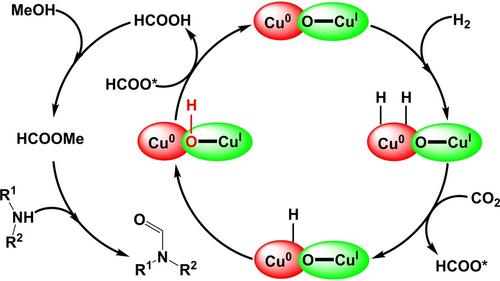
The proposed reaction mechanism.
Conclusion
In summary, the challenging N-formylation of aliphatic primary amines with CO2/H2 was achieved by catalysts containing Cu2O/Cu interface sites. The introduction of an aluminum oxide support strongly stabilizes the active mixed valence Cu2O/Cu structure, and the catalyst can be consecutively used for 6 times without obvious activity loss. A series of formamides with diverse structures was synthesized in high yields. In situ XRD, pseudo in situ XPS, HAADF-STEM and ELNES characterization results confirmed the co-existence of both Cu2O and Cu sites in the active catalysts, and a study of the reaction mechanism showed that methyl formate is the possible reaction intermediate. DFT calculation revealed that, compared with a pure Cu(111) surface, CO2 conversion on the Cu2O/Cu(111) interface was energetically more favorable, since oxygen from the Cu2O/Cu interface, by forming Ointer-H, does not only efficiently reduce the apparent barrier of HCOOH formation but also facilitates desorption of the N-formylated amine from the Cu2O/Cu interface, affording high activity and selectivity.
Acknowledgments
The National Natural Sciences Foundation of China (21961132025, 21925207, and 22102199), Gansu Science and Technology Major Project (21ZD4WA021, 22ZD6GA003 and 21JR7RA096), CAS Project for Young Scientists in Basic Research (Grant No. YSBR-050), Key Program of the Lanzhou Institute of Chemical Physics, CAS (No. KJZLZD-2) and Deutsche Forschungsgemeinschaft (grant no. BR 1380/27-1) are gratefully acknowledged. We thank Dr. Dongmei Li and Li He (Lanzhou Institute of Chemical Physics, CAS) for helping us to perform XRD characterization. Funding from the German Federal Ministry for Education and Research for the electron energy loss spectrometer within the program ESMAC is kindly acknowledged. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.