Rhodium(I) Carbene-Promoted Enantioselective C−H Functionalization of Simple Unprotected Indoles, Pyrroles and Heteroanalogues: New Mechanistic Insights
Tian-Yi Wang
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, and School of Pharmacy, University of Chinese Academy of Sciences, Shanghai, 201203 China
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
These authors contributed equally to this work.
Search for more papers by this authorXiao-Xuan Chen
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Dong-Xing Zhu
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, and School of Pharmacy, University of Chinese Academy of Sciences, Shanghai, 201203 China
Search for more papers by this authorProf. Dr. Lung Wa Chung
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Ming-Hua Xu
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, and School of Pharmacy, University of Chinese Academy of Sciences, Shanghai, 201203 China
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan 453007 China
Search for more papers by this authorTian-Yi Wang
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, and School of Pharmacy, University of Chinese Academy of Sciences, Shanghai, 201203 China
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
These authors contributed equally to this work.
Search for more papers by this authorXiao-Xuan Chen
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Dong-Xing Zhu
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, and School of Pharmacy, University of Chinese Academy of Sciences, Shanghai, 201203 China
Search for more papers by this authorProf. Dr. Lung Wa Chung
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Ming-Hua Xu
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, and School of Pharmacy, University of Chinese Academy of Sciences, Shanghai, 201203 China
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan 453007 China
Search for more papers by this authorGraphical Abstract
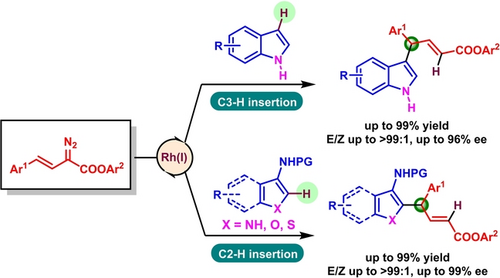
A highly enantioselective C(sp2)−H functionalization of simple unprotected indoles, pyrroles, and their common analogues such as furans, thiophenes, and benzofurans by arylvinylcarbene insertion to access unique heteroarene-containing chiral diarylmethine derivatives has been developed for the first time. Mechanistic and DFT calculation studies revealed that the reductive elimination is the rate-determining step.
Abstract
A rhodium(I)-diene catalyzed highly enantioselective C(sp2)−H functionalization of simple unprotected indoles, pyrroles, and their common analogues such as furans, thiophenes, and benzofurans with arylvinyldiazoesters has been developed for the first time. This transformation features unusual site-selectivity exclusively at the vinyl terminus of arylvinylcarbene and enables a reliable and rapid synthetic protocol to access a distinctive class of diarylmethine-bearing α,β-unsaturated esters containing a one or two heteroarene-attached tertiary carbon stereocenter in high yields and excellent enantioselectivities under mild reaction conditions. Mechanistic studies and DFT calculations suggest that, compared to the aniline substrate, the more electron-rich indole substrate lowers the C−C addition barrier and alters the rate-determining step to the reductive elimination, leading to different isotope effect.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202207008-sup-0001-3af.cif275 KB | Supporting Information |
| anie202207008-sup-0001-5n.cif352.8 KB | Supporting Information |
| anie202207008-sup-0001-6b.cif459.1 KB | Supporting Information |
| anie202207008-sup-0001-misc_information.pdf11.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews, see:
- 1aD. Ameen, T. J. Snape, MedChemComm 2013, 4, 893–907;
- 1bT. Jia, P. Gao, J. Liao, Chem. Sci. 2018, 9, 546–559;
- 1cM. Sankalan, R. Deblina, P. Gautam, ChemCatChem 2018, 10, 1941–1967;
- 1dM. Belal, Z. Li, X. Lu, G. Yin, Sci. Chi. Chem. 2021, 64, 513–533.
- 2For recent selected examples, see:
- 2aM. Prat, D. Fernández, M. A. Buil, M. I. Crespo, G. Casals, M. Ferrer, L. Tort, J. Castro, J. M. Monleón, A. Gavaldà, M. Miralpeix, I. Ramos, T. Doménech, D. Vilella, F. Antón, J. M. Huerta, S. Espinosa, M. López, S. Sentellas, M. González, J. Albertí, V. Segarra, A. Cárdenas, J. Beleta, H. Ryder, J. Med. Chem. 2009, 52, 5076–5092;
- 2bL. D. Pennington, M. D. Bartberger, M. D. Croghan, K. L. Andrews, K. S. Ashton, M. P. Bourbeau, J. Chen, S. Chmait, R. Cupples, C. Fotsch, J. Helmering, F.-T. Hong, R. W. Hungate, S. R. Jordan, K. Kong, L. Liu, K. Michelsen, C. Moyer, N. Nishimura, M. H. Norman, A. Reichelt, A. C. Siegmund, G. Sivits, S. Tadesse, C. M. Tegley, G. Van, K. C. Yang, G. Yao, J. Zhang, D. J. Lloyd, C. Hale, D. J. St. Jean, J. Med. Chem. 2015, 58, 9663–9679;
- 2cM. S. Islam, A. Barakat, A. M. Al-Majid, M. Ali, S. Yousuf, M. I. Choudhary, R. Khalil, Z. Ul-Haq, Bioorg. Chem. 2018, 79, 350–354;
- 2dC.-C. Tseng, G. Baillie, G. Donvito, M.-A. Mustafa, S.-E. Juola, C. Zanato, C. Massarenti, S. Dall'Angelo, W. T. A. Harrison, A.-H. Lichtman, R.-A. Ross, M. Zanda, I.-R. Greig, J. Med. Chem. 2019, 62, 5049–5062;
- 2eY.-L. Zou, H.-X. Li, E.-T. Graham, A.-A. Deik, J.-K. Eaton, W.-L. Wang, S.-G. Gerardo, C.-B. Clish, J.-G. Doench, S.-L. Schreiber, Nat. Chem. Biol. 2020, 16, 302–309;
- 2fC. Eurtivong, I.-P. Lisa, M.-V. Rensburg, R.-M. White, H.-K. Bara, S. Rees, K.-P. Emily, C.-S. Xu, N. Sharma, I. K. H. Leung, E. Leung, D. Barker, J. Reynisson, Eur. J. Med. Chem. 2020, 187, 111919.
- 3For reviews on C−H functionalization by carbene insertion, see:
- 3aH. M. L. Davies, R. E. J. Beckwith, Chem. Rev. 2003, 103, 2861–2903;
- 3bM. M. Díaz-Requejo, P. J. Pérez, Chem. Rev. 2008, 108, 3379–3394;
- 3cH. M. L. Davies, J. R. Manning, Nature 2008, 451, 417–424;
- 3dM. P. Doyle, R. Duffy, M. Ratnikov, L. Zhou, Chem. Rev. 2010, 110, 704–724;
- 3eH. M. L. Davies, D. Morton, Chem. Soc. Rev. 2011, 40, 1857–1869;
- 3fS.-F. Zhu, Q.-L. Zhou, Acc. Chem. Res. 2012, 45, 1365–1377;
- 3gA. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire, M. A. McKervey, Chem. Rev. 2015, 115, 9981–10080.
- 4For recent reviews on transition-metal-catalyzed enantioselective indole functionalizations, see:
- 4aD. Xing, W. Hu, Tetrahedron Lett. 2014, 55, 777–783;
- 4bJ.-B. Chen, Y.-X. Jia, Org. Biomol. Chem. 2017, 15, 3550–3567;
- 4cY.-P. Li, Z.-Q. Li, S.-F. Zhu, Tetrahedron Lett. 2018, 59, 2307–2316.
- 5Y. Lian, H. M. L. Davies, J. Am. Chem. Soc. 2010, 132, 440–441.
- 6Y. Cai, S.-F. Zhu, G.-P. Wang, Q.-L. Zhou, Adv. Synth. Catal. 2011, 353, 2939–2944.
- 7X. Gao, B. Wu, W.-X. Huang, M.-W. Chen, Y.-G. Zhou, Angew. Chem. Int. Ed. 2015, 54, 11956–11960; Angew. Chem. 2015, 127, 12124–12128.
- 8X. Gao, B. Wu, Z. Yam, Y.-G. Zhou, Org. Biomol. Chem. 2016, 14, 8237–8240.
- 9N. Li, W.-J. Zhu, J.-J. Huang, X.-Q. Hao, J.-F. Gong, M.-P. Song, Organometallics 2020, 39, 2222–2234.
- 10A. N. Leveille, R. Echemendía, A. E. Mattson, A. C. B. Burtoloso, Org. Lett. 2021, 23, 9446–9450.
- 11For two successful examples of asymmetric C3−H functionalization with the use of unprotected indoles, see:
- 11aW. Yang, X. Lin, Y. Zhang, W. Cao, X. Liu, X. Feng, Chem. Commun. 2020, 56, 10002–10005;
- 11bS. Dong, X. Liu, X. Feng, Acc. Chem. Res. 2022, 55, 415–428; and ref. [10]. For two non-asymmetric examples, see:
- 11cJ. Shin, K. B. S. Magar, J. Lee, K.-S. Kim, Y. R. Lee, Sci. Rep. 2019, 9, 8012–8029;
- 11dA. Dasgupta, R. Babaahmadi, B. Slater, B. F. Yates, A. Ariafard, R. L. Melen, Chem 2020, 6, 2364–2381.
- 12For non-asymmetric C2−H functionalization examples, see:
- 12aW.-W. Chan, S.-H. Yeung, Z.-Y. Zhou, A. S. C. Chan, W.-Y. Yu, Org. Lett. 2010, 12, 604–607;
- 12bM. J. James, P. O'Brien, R. J. K. Taylor, W. P. Unsworth, Angew. Chem. Int. Ed. 2016, 55, 9671–9675; Angew. Chem. 2016, 128, 9823–9827;
- 12cJ. Ghorai, M. Chaitanya, P. Anbarasan, Org. Biomol. Chem. 2018, 16, 7346–7350;
- 12dE. Nag, S. M. N. V. T. Gorantla, S. Arumugam, A. Kulkarni, K. C. Mondal, S. Roy, Org. Lett. 2020, 22, 6313–6318;
- 12eS. Guha, S. Gadde, N. Kumar, D. S. Black, S. Sen, J. Org. Chem. 2021, 86, 5234–5244. For examples of N−H insertion of indoles, see:
- 12fV. Arredondo, S. C. Hiew, E. S. Gutman, I. D. U. A. Premachandra, D. L. V. Vranken, Angew. Chem. Int. Ed. 2017, 56, 4156–4159; Angew. Chem. 2017, 129, 4220–4223;
- 12gD. Maiti, R. Das, S. Sen, J. Org. Chem. 2021, 86, 2522–2533.
- 13
- 13aD. Chen, X. Zhang, W.-Y. Qi, B. Xu, M.-H. Xu, J. Am. Chem. Soc. 2015, 137, 5268–5271;
- 13bD. Chen, D.-X. Zhu, M.-H. Xu, J. Am. Chem. Soc. 2016, 138, 1498–1501;
- 13cB. Liu, M.-H. Xu, Chin. J. Chem. 2021, 39, 1911–1915;
- 13dY.-T. Sun, X. Rao, W. Xu, M.-H. Xu, Org. Chem. Front. 2022, 9, 3467–3472.
- 14
- 14aD.-X. Zhu, H. Xia, J.-G. Liu, L. W. Chung, M.-H. Xu, J. Am. Chem. Soc. 2021, 143, 2608–2619;
- 14bD.-X. Zhu, J.-G. Liu, M.-H. Xu, J. Am. Chem. Soc. 2021, 143, 8583–8589.
- 15Deposition Numbers 2170118 (for 3af), 2170119 (for 5n), and 2170120 (for 6b) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 16
- 16aD. Zhang, H. Qiu, L. Jiang, F. Lv, C. Ma, W. Hu, Angew. Chem. Int. Ed. 2013, 52, 13356–13360; Angew. Chem. 2013, 125, 13598–13602;
- 16bZ.-Y. Cao, Y.-L. Zhao, J. Zhou, Chem. Commun. 2016, 52, 2537–2540;
- 16cH.-Q. Shen, C. Liu, J. Zhou, Y.-G. Zhou, Org. Chem. Front. 2018, 5, 611–614.
- 17Y. Lian, H. M. L. Davies, Org. Lett. 2012, 14, 1934–1937.
- 18See Supporting Information for computational details.
- 19Selected DFT studies on reactions of transition metal-carbene complexes:
- 19aE. Nakamura, N. Yoshikai, M. Yamanaka, J. Am. Chem. Soc. 2002, 124, 7181–7192;
- 19bJ. H. Hansen, H. M. L. Davies, Chem. Sci. 2011, 2, 457–461;
- 19cJ. H. Hansen, T. M. Gregg, S. R. Ovalles, Y. Lian, J. Autschbach, H. M. L. Davies, J. Am. Chem. Soc. 2011, 133, 5076–5085;
- 19dY. Liu, Z. Yu, J.-Z. Zhang, L. Liu, F. Xia, J. Zhang, Chem. Sci. 2016, 7, 1988–1995;
- 19eD. Solé, F. Mariani, M. L. Bennasar, I. Fernández, Angew. Chem. Int. Ed. 2016, 55, 6467–6470; Angew. Chem. 2016, 128, 6577–6580;
- 19fS. R. Hare, D. J. Tantillo, Chem. Sci. 2017, 8, 1442–1449;
- 19gS.-Y. Liu, J. Jiang, J.-H. Chen, Q.-H. Wei, W.-F. Yao, F. Xia, W.-H. Hu, Chem. Sci. 2017, 8, 4312–4317;
- 19hH.-S. Xie, Z.-R. Ye, Z.-F. Ke, J.-Y. Lan, H.-F. Jiang, W. Zeng, Chem. Sci. 2018, 9, 985–989;
- 19iL.-J. Song, Q. Feng, Y. Wang, S.-T. Ding, Y.-D. Wu, X.-H. Zhang, L. W. Chung, J.-W. Sun, J. Am. Chem. Soc. 2019, 141, 17441–17451;
- 19jL.-L. Yang, D. Evans, B. Xu, W.-T. Li, M.-L. Li, S.-F. Zhu, K. N. Houk, Q.-L. Zhou, J. Am. Chem. Soc. 2020, 142, 12394–12399;
- 19kW. Yang, M.-P. Pu, X.-B. Lin, M. Chen, Y.-J. Song, X.-H. Liu, Y.-D. Wu, X.-M. Feng, J. Am. Chem. Soc. 2021, 143, 9648–9656;
- 19lJ.-L. Lan, X. Li, Y.-H. Yang, X.-Y. Zhang, L. W. Chung, Acc. Chem. Res. 2022, 55, 1109–1123.
- 20
- 20aE. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen, W. Yang, J. Am. Chem. Soc. 2010, 132, 6498–6506;
- 20bR. F. W. Bader, Atoms in Molecules: A Quantum Theory, Clarendon Press, Oxford, 1990.
- 21
- 21aF. M. Bickelhaupt, K. N. Houk, Angew. Chem. Int. Ed. 2017, 56, 10070–10086; Angew. Chem. 2017, 129, 10204–10221;
- 21bC.-Y. Chen, Z.-F. Zhang, S.-C. Jin, X.-R. Fan, M.-Y. Geng, Y. Zhou, S.-W. Wen, X.-R. Wang, L. W. Chung, X.-Q. Dong, X.-M. Zhang, Angew. Chem. Int. Ed. 2017, 56, 6808–6812; Angew. Chem. 2017, 129, 6912–6916.
- 22M. Gómez-Gallego, M. A. Sierra, Chem. Rev. 2011, 111, 4857–4963.