Highly Active and Stable Li2S−Cu Nanocomposite Cathodes Enabled by Kinetically Favored Displacement Interconversion between Cu2S and Li2S
Graphical Abstract
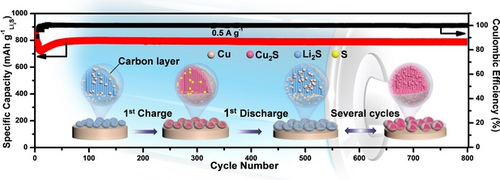
A nanostructured Li2S−Cu composite is fabricated and it is demonstrated that Cu progressively participates in the redox processes and fundamentally alters the redox couple from anion-type Li2S/S to cation-type Cu/Cu2S, which leads to a drastically reduced activation potential (2.35 V vs. Li+/Li), superior rate capability, and stable long-term cycling performance.
Abstract
The high activation barrier, inferior rate performance, and short cycling life severely constrain the practical applications of the high-capacity Li2S cathode. Herein, we fabricate a Li2S−Cu nanocomposite with a drastically reduced activation potential, fast rate capability, and extraordinary cycling stability even under a practically relevant areal capacity of 2.96 mAh cm−2. Detailed experimental investigations aided by theoretical calculations indicate that instead of converting to S8 via troublesome soluble lithium polysulfides, Li2S is thermodynamically and kinetically more favorable to react with Cu by the displacement reaction, which alters the redox couple from Li2S/S to Cu/Cu2S, leading to the excellent electrochemical performance. Moreover, the stability of the composite is demonstrated in the full-cell configuration consisting of commercial graphite anodes. This work provides an innovative and effective approach to realize highly activated and stable Li2S cathode materials.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.