Stabilization of Cu+ via Strong Electronic Interaction for Selective and Stable CO2 Electroreduction
Yixiang Zhou
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorYebo Yao
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorRui Zhao
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorXiaoxuan Wang
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorZhenzhen Fu
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorDewei Wang
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorHuaizhi Wang
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorLiang Zhao
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorWei Ni
Beijing Aerospace Propulsion Institute, Beijing, 100076 China
Search for more papers by this authorCorresponding Author
Zhiyu Yang
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorCorresponding Author
Yi-Ming Yan
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorYixiang Zhou
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorYebo Yao
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorRui Zhao
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorXiaoxuan Wang
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorZhenzhen Fu
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorDewei Wang
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorHuaizhi Wang
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorLiang Zhao
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorWei Ni
Beijing Aerospace Propulsion Institute, Beijing, 100076 China
Search for more papers by this authorCorresponding Author
Zhiyu Yang
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorCorresponding Author
Yi-Ming Yan
State Key Lab of Organic-Inorganic Composites, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorGraphical Abstract
Abstract
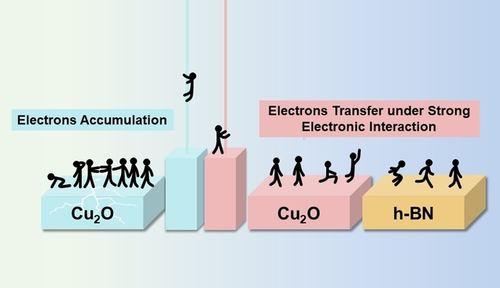
Copper oxide-based materials effectively electrocatalyze carbon dioxide reduction (CO2RR). To comprehend their role and achieve high CO2RR activity, Cu+ in copper oxides must be stabilized. As an electrocatalyst, Cu2O nanoparticles were decorated with hexagonal boron nitride (h-BN) nanosheets to stabilize Cu+. The C2H4/CO ratio increased 1.62-fold in the CO2RR with Cu2O−BN compared to that with Cu2O. Experimental and theoretical studies confirmed strong electronic interactions between the two components in Cu2O−BN, which strengthens the Cu−O bonds. Electrophilic h-BN receives partial electron density from Cu2O, protecting the Cu−O bonds from electron attack during the CO2RR and stabilizing the Cu+ species during long-term electrolysis. The well-retained Cu+ species enhanced the C2 product selectivity and improved the stability of Cu2O−BN. This work offers new insight into the metal-valence-state-dependent selectivity of catalysts, enabling the design of advanced catalysts.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202205832-sup-0001-Cu2O(111).cif5.9 KB | Supporting Information |
| anie202205832-sup-0001-Cu2O-BN.cif8.3 KB | Supporting Information |
| anie202205832-sup-0001-h-BN(002).cif3.4 KB | Supporting Information |
| anie202205832-sup-0001-misc_information.pdf4.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Y. Y. Birdja, E. Pérez-Gallent, M. C. Figueiredo, A. J. Göttle, F. Calle-Vallejo, M. T. M. Koper, Nat. Energy 2019, 4, 732–745.
- 2L. Zhang, Z.-J. Zhao, J. Gong, Angew. Chem. Int. Ed. 2017, 56, 11326–11353; Angew. Chem. 2017, 129, 11482–11511.
- 3G. Wen, B. Ren, M. G. Park, J. Yang, H. Dou, Z. Zhang, Y.-P. Deng, Z. Bai, L. Yang, J. Gostick, G. A. Botton, Y. Hu, Z. Chen, Angew. Chem. Int. Ed. 2020, 59, 12860–12867; Angew. Chem. 2020, 132, 12960–12967.
- 4L.-P. Yuan, W.-J. Jiang, X.-L. Liu, Y.-H. He, C. He, T. Tang, J. Zhang, J.-S. Hu, ACS Catal. 2020, 10, 13227–13235.
- 5S. Nitopi, E. Bertheussen, S. B. Scott, X. Liu, A. K. Engstfeld, S. Horch, B. Seger, I. E. L. Stephens, K. Chan, C. Hahn, J. K. Nørskov, T. F. Jaramillo, I. Chorkendorff, Chem. Rev. 2019, 119, 7610–7672.
- 6G. M. Tomboc, S. Choi, T. Kwon, Y. J. Hwang, K. Lee, Adv. Mater. 2020, 32, 1908398.
- 7Y. Hori in Modern Aspects of Electrochemistry (Eds.: C. G. Vayenas, R. E. White, M. E. Gamboa-Aldeco), Springer New York, New York, 2008, pp. 89–189.
10.1007/978-0-387-49489-0_3 Google Scholar
- 8H. Xie, T. Wang, J. Liang, Q. Li, S. Sun, Nano Today 2018, 21, 41–54.
- 9W. Ni, C. Li, X. Zang, M. Xu, S. Huo, M. Liu, Z. Yang, Y.-M. Yan, Appl. Catal. B 2019, 259, 118044.
- 10K. P. Kuhl, E. R. Cave, D. N. Abram, T. F. Jaramillo, Energy Environ. Sci. 2012, 5, 7050–7059.
- 11Y. Wang, J. Liu, G. Zheng, Adv. Mater. 2021, 33, 2005798.
- 12Z.-Z. Wu, F.-Y. Gao, M.-R. Gao, Energy Environ. Sci. 2021, 14, 1121–1139.
- 13J. Wang, H.-Y. Tan, Y. Zhu, H. Chu, H. M. Chen, Angew. Chem. Int. Ed. 2021, 60, 17254–17267; Angew. Chem. 2021, 133, 17394–17407.
- 14R. M. Arán-Ais, F. Scholten, S. Kunze, R. Rizo, B. Roldan Cuenya, Nat. Energy 2020, 5, 317–325.
- 15Z. Yin, C. Yu, Z. Zhao, X. Guo, M. Shen, N. Li, M. Muzzio, J. Li, H. Liu, H. Lin, J. Yin, G. Lu, D. Su, S. Sun, Nano Lett. 2019, 19, 8658–8663.
- 16S.-C. Lin, C.-C. Chang, S.-Y. Chiu, H.-T. Pai, T.-Y. Liao, C.-S. Hsu, W.-H. Chiang, M.-K. Tsai, H. M. Chen, Nat. Commun. 2020, 11, 3525.
- 17M. Favaro, H. Xiao, T. Cheng, W. A. Goddard, J. Yano, E. J. Crumlin, Proc. Natl. Acad. Sci. USA 2017, 114, 6706–6711.
- 18X. Bai, Q. Li, L. Shi, X. Niu, C. Ling, J. Wang, Small 2020, 16, 1901981.
- 19J. Jiao, R. Lin, S. Liu, W.-C. Cheong, C. Zhang, Z. Chen, Y. Pan, J. Tang, K. Wu, S.-F. Hung, H. M. Chen, L. Zheng, Q. Lu, X. Yang, B. Xu, H. Xiao, J. Li, D. Wang, Q. Peng, C. Chen, Y. Li, Nat. Chem. 2019, 11, 222–228.
- 20X. Yuan, S. Chen, D. Cheng, L. Li, W. Zhu, D. Zhong, Z.-J. Zhao, J. Li, T. Wang, J. Gong, Angew. Chem. Int. Ed. 2021, 60, 15344–15347; Angew. Chem. 2021, 133, 15472–15475.
- 21C. Kim, K. M. Cho, K. Park, J. Y. Kim, G.-T. Yun, F. M. Toma, I. Gereige, H.-T. Jung, Adv. Funct. Mater. 2021, 31, 2102142.
- 22Q. Fan, X. Zhang, X. Ge, L. Bai, D. He, Y. Qu, C. Kong, J. Bi, D. Ding, Y. Cao, X. Duan, J. Wang, J. Yang, Y. Wu, Adv. Energy Mater. 2021, 11, 2101424.
- 23H. Xu, D. Rebollar, H. He, L. Chong, Y. Liu, C. Liu, C.-J. Sun, T. Li, J. V. Muntean, R. E. Winans, D.-J. Liu, T. Xu, Nat. Energy 2020, 5, 623–632.
- 24H. Xiao, W. A. Goddard, T. Cheng, Y. Liu, Proc. Natl. Acad. Sci. USA 2017, 114, 6685–6688.
- 25T.-C. Chou, C.-C. Chang, H.-L. Yu, W.-Y. Yu, C.-L. Dong, J.-J. Velasco-Vélez, C.-H. Chuang, L.-C. Chen, J.-F. Lee, J.-M. Chen, H.-L. Wu, J. Am. Chem. Soc. 2020, 142, 2857–2867.
- 26S. H. Lee, J. C. Lin, M. Farmand, A. T. Landers, J. T. Feaster, J. E. Avilés Acosta, J. W. Beeman, Y. Ye, J. Yano, A. Mehta, R. C. Davis, T. F. Jaramillo, C. Hahn, W. S. Drisdell, J. Am. Chem. Soc. 2021, 143, 588–592.
- 27J.-J. Velasco-Velez, R. V. Mom, L.-E. Sandoval-Diaz, L. J. Falling, C.-H. Chuang, D. Gao, T. E. Jones, Q. Zhu, R. Arrigo, B. Roldan Cuenya, A. Knop-Gericke, T. Lunkenbein, R. Schlögl, ACS Energy Lett. 2020, 5, 2106–2111.
- 28W. Ma, S. Xie, T. Liu, Q. Fan, J. Ye, F. Sun, Z. Jiang, Q. Zhang, J. Cheng, Y. Wang, Nat. Catal. 2020, 3, 478–487.
- 29H. Li, T. Liu, P. Wei, L. Lin, D. Gao, G. Wang, X. Bao, Angew. Chem. Int. Ed. 2021, 60, 14329–14333; Angew. Chem. 2021, 133, 14450–14454.
- 30C. Chen, X. Sun, L. Lu, D. Yang, J. Ma, Q. Zhu, Q. Qian, B. Han, Green Chem. 2018, 20, 4579–4583.
- 31Y. Zhou, F. Che, M. Liu, C. Zou, Z. Liang, P. De Luna, H. Yuan, J. Li, Z. Wang, H. Xie, H. Li, P. Chen, E. Bladt, R. Quintero-Bermudez, T.-K. Sham, S. Bals, J. Hofkens, D. Sinton, G. Chen, E. H. Sargent, Nat. Chem. 2018, 10, 974–980.
- 32J. Li, A. Ozden, M. Wan, Y. Hu, F. Li, Y. Wang, R. R. Zamani, D. Ren, Z. Wang, Y. Xu, D.-H. Nam, J. Wicks, B. Chen, X. Wang, M. Luo, M. Graetzel, F. Che, E. H. Sargent, D. Sinton, Nat. Commun. 2021, 12, 2808.
- 33S. Chu, X. Yan, C. Choi, S. Hong, A. W. Robertson, J. Masa, B. Han, Y. Jung, Z. Sun, Green Chem. 2020, 22, 6540–6546.
- 34P. De Luna, R. Quintero-Bermudez, C.-T. Dinh, M. B. Ross, O. S. Bushuyev, P. Todorović, T. Regier, S. O. Kelley, P. Yang, E. H. Sargent, Nat. Catal. 2018, 1, 103–110.
- 35P.-P. Yang, X.-L. Zhang, F.-Y. Gao, Y.-R. Zheng, Z.-Z. Niu, X. Yu, R. Liu, Z.-Z. Wu, S. Qin, L.-P. Chi, Y. Duan, T. Ma, X.-S. Zheng, J.-F. Zhu, H.-J. Wang, M.-R. Gao, S.-H. Yu, J. Am. Chem. Soc. 2020, 142, 6400–6408.
- 36N. Sakamoto, Y. F. Nishimura, T. Nonaka, M. Ohashi, N. Ishida, K. Kitazumi, Y. Kato, K. Sekizawa, T. Morikawa, T. Arai, ACS Catal. 2020, 10, 10412–10419.
- 37W. Wang, C. Deng, S. Xie, Y. Li, W. Zhang, H. Sheng, C. Chen, J. Zhao, J. Am. Chem. Soc. 2021, 143, 2984–2993.
- 38X. Wu, Y. Guo, Z. Sun, F. Xie, D. Guan, J. Dai, F. Yu, Z. Hu, Y.-C. Huang, C.-W. Pao, J.-L. Chen, W. Zhou, Z. Shao, Nat. Commun. 2021, 12, 660.
- 39S. Popović, M. Smiljanić, P. Jovanovič, J. Vavra, R. Buonsanti, N. Hodnik, Angew. Chem. Int. Ed. 2020, 59, 14736–14746; Angew. Chem. 2020, 132, 14844–14854.
- 40B. Zhao, B. Yan, Z. Jiang, S. Yao, Z. Liu, Q. Wu, R. Ran, S. D. Senanayake, D. Weng, J. G. Chen, Chem. Commun. 2018, 54, 7354–7357.
- 41C. Huang, W. Ye, Q. Liu, X. Qiu, ACS Appl. Mater. Interfaces 2014, 6, 14469–14476.
- 42A. Nag, K. Raidongia, K. P. S. S. Hembram, R. Datta, U. V. Waghmare, C. N. R. Rao, ACS Nano 2010, 4, 1539–1544.
- 43R. Arenal, A. C. Ferrari, S. Reich, L. Wirtz, J. Y. Mevellec, S. Lefrant, A. Rubio, A. Loiseau, Nano Lett. 2006, 6, 1812–1816.
- 44M. Balkanski, M. A. Nusimovici, J. Reydellet, Solid State Commun. 1969, 7, 815–818.
- 45W. Bi, Y. Hu, H. Jiang, L. Zhang, C. Li, Adv. Funct. Mater. 2021, 31, 2010780.
- 46W. Zhang, X. Shi, Z. Yan, Y. Shan, Y. Zhu, Y. Yu, H. He, ACS Catal. 2021, 11, 9825–9836.
- 47W. Lei, D. Portehault, R. Dimova, M. Antonietti, J. Am. Chem. Soc. 2011, 133, 7121–7127.
- 48L. Ci, L. Song, C. Jin, D. Jariwala, D. Wu, Y. Li, A. Srivastava, Z. F. Wang, K. Storr, L. Balicas, F. Liu, P. M. Ajayan, Nat. Mater. 2010, 9, 430–435.
- 49A. Agresti, A. Pazniak, S. Pescetelli, A. Di Vito, D. Rossi, A. Pecchia, M. Auf der Maur, A. Liedl, R. Larciprete, D. V. Kuznetsov, D. Saranin, A. Di Carlo, Nat. Mater. 2019, 18, 1228–1234.
- 50H. An, L. Wu, L. D. B. Mandemaker, S. Yang, J. de Ruiter, J. H. J. Wijten, J. C. L. Janssens, T. Hartman, W. van der Stam, B. M. Weckhuysen, Angew. Chem. Int. Ed. 2021, 60, 16576–16584; Angew. Chem. 2021, 133, 16712–16720.
- 51P. Y. Yu, Y. R. Shen, Y. Petroff, Solid State Commun. 1973, 12, 973–975.
- 52P. Y. Yu, Y. R. Shen, Phys. Rev. B 1975, 12, 1377–1394.
- 53G. Liu, M. Lee, S. Kwon, G. Zeng, J. Eichhorn, A. K. Buckley, F. D. Toste, W. A. Goddard, F. M. Toma, Proc. Natl. Acad. Sci. USA 2021, 118, e2012649118.
- 54J. Duan, S. Chen, C. A. Ortíz-Ledón, M. Jaroniec, S.-Z. Qiao, Angew. Chem. Int. Ed. 2020, 59, 8181–8186; Angew. Chem. 2020, 132, 8258–8263.
- 55G. Hu, Z. Wu, S. Dai, D.-e. Jiang, ACS Appl. Mater. Interfaces 2018, 10, 6694–6700.
- 56M. Xue, M. Nakayama, P. Liu, M. G. White, J. Phys. Chem. C 2017, 121, 22234–22247.