Molecular Engineering of Metal Complexes for Electrocatalytic Carbon Dioxide Reduction: From Adjustment of Intrinsic Activity to Molecular Immobilization
Zhi-Wen Yang
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorJin-Mei Chen
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorLi-Qi Qiu
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorWen-Jun Xie
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Liang-Nian He
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorZhi-Wen Yang
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorJin-Mei Chen
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorLi-Qi Qiu
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorWen-Jun Xie
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Liang-Nian He
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorGraphical Abstract
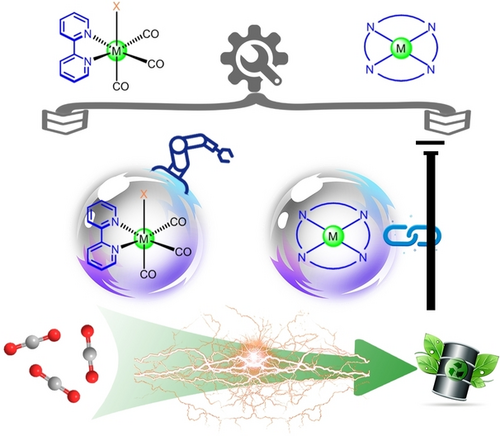
This Review focuses on molecular catalysts for the electrocatalytic CO2 reduction reaction (ECO2RR) including metal bipyridines and macrocycle complexes, and summarizes the molecular engineering strategies developed to regulate the intrinsic catalytic efficiency and modify the electrode. Guidelines are provided for the rational design of ECO2RR catalytic systems.
Abstract
The electrocatalytic CO2 reduction reaction (ECO2RR) is one promising method for storing intermittent clean energy in chemical bonds and producing fuels. Among various kinds of catalysts for ECO2RR, molecular metal complexes with well-defined structures are convenient for studies of their rational design, structure–reactivity relationships, and mechanisms. In this Review, we summarize the molecular engineering of several N-based metal complexes including Re/Mn bipyridine compounds and metal macrocycles, concluding with general modification strategies to devise novel molecular catalysts with high intrinsic activity. Through physical adsorption, covalent linking, and formation of a periodic backbone, these active molecules can be heterogenized into immobilized catalysts with more practical prospects. Finally, significant challenges and opportunities based on molecular catalysts are discussed.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1
- 1aD. U. Hooper, E. C. Adair, B. J. Cardinale, J. E. Byrnes, B. A. Hungate, K. L. Matulich, A. Gonzalez, J. E. Duffy, L. Gamfeldt, M. I. O′Connor, Nature 2012, 486, 105–108;
- 1bS. S. Myers, A. Zanobetti, I. Kloog, P. Huybers, A. D. Leakey, A. J. Bloom, E. Carlisle, L. H. Dietterich, G. Fitzgerald, T. Hasegawa, N. M. Holbrook, R. L. Nelson, M. J. Ottman, V. Raboy, H. Sakai, K. A. Sartor, J. Schwartz, S. Seneweera, M. Tausz, Y. Usui, Nature 2014, 510, 139–142;
- 1cW. Cai, S. Borlace, M. Lengaigne, P. van Rensch, M. Collins, G. Vecchi, A. Timmermann, A. Santoso, M. J. McPhaden, L. Wu, M. H. England, G. Wang, E. Guilyardi, F.-F. Jin, Nat. Clim. Change 2014, 4, 111–116.
- 2O. S. Bushuyev, P. De Luna, C. T. Dinh, L. Tao, G. Saur, J. van de Lagemaat, S. O. Kelley, E. H. Sargent, Joule 2018, 2, 825–832.
- 3A. Beck, M. Zabilskiy, M. A. Newton, O. Safonova, M. G. Willinger, J. A. van Bokhoven, Nat. Catal. 2021, 4, 488–497.
- 4
- 4aA. Alissandratos, C. J. Easton, Beilstein J. Org. Chem. 2015, 11, 2370–2387;
- 4bM. Baccour, A. Lamotte, K. Sakai, E. Dubreucq, A. Mehdi, K. Kano, A. Galarneau, J. Drone, N. Brun, Green Chem. 2020, 22, 3727–3733.
- 5F. Franco, C. Rettenmaier, H. S. Jeon, B. Roldan Cuenya, Chem. Soc. Rev. 2020, 49, 6884–6946.
- 6
- 6aK.-H. Chen, N. Wang, Z.-W. Yang, S.-M. Xia, L.-N. He, ChemSusChem 2020, 13, 6284–6289;
- 6bL.-Q. Qiu, K.-H. Chen, Z.-W. Yang, L.-N. He, Green Chem. 2020, 22, 8614–8622.
- 7J. Chen, J. Yin, X. Zheng, H. Ait Ahsaine, Y. Zhou, C. Dong, O. F. Mohammed, K. Takanabe, O. M. Bakr, ACS Energy Lett. 2019, 4, 1279–1286.
- 8A. Álvarez, M. Borges, J. J. Corral-Pérez, J. G. Olcina, L. Hu, D. Cornu, R. Huang, D. Stoian, A. Urakawa, ChemPhysChem 2017, 18, 3135–3141.
- 9R. Francke, B. Schille, M. Roemelt, Chem. Rev. 2018, 118, 4631–4701.
- 10M. B. Ross, P. De Luna, Y. Li, C.-T. Dinh, D. Kim, P. Yang, E. H. Sargent, Nat. Catal. 2019, 2, 648–658.
- 11
- 11aN. Elgrishi, M. B. Chambers, X. Wang, M. Fontecave, Chem. Soc. Rev. 2017, 46, 761–796;
- 11bJ.-W. Wang, W.-J. Liu, D.-C. Zhong, T.-B. Lu, Coord. Chem. Rev. 2019, 378, 237–261;
- 11cL.-M. Cao, H.-H. Huang, J.-W. Wang, D.-C. Zhong, T.-B. Lu, Green Chem. 2018, 20, 798–803;
- 11dJ. W. Wang, H. H. Huang, J. K. Sun, T. Ouyang, D. C. Zhong, T. B. Lu, ChemSusChem 2018, 11, 1025–1031.
- 12
- 12aD. K. Yadav, D. K. Singh, V. Ganesan, Curr. Opin. Electrochem. 2020, 22, 87–93;
- 12bM. Li, H. Wang, W. Luo, P. C. Sherrell, J. Chen, J. Yang, Adv. Mater. 2020, 32, 2001848.
- 13A. C. Pérez-Sequera, M. A. Díaz-Pérez, J. C. Serrano-Ruiz, Catalysts 2020, 10, 1179.
- 14S. Zhang, Q. Fan, R. Xia, T. J. Meyer, Acc. Chem. Res. 2020, 53, 255–264.
- 15B. B. Zhang, L. Z. Fan, R. B. Ambre, T. Q. Liu, Q. J. Meng, B. J. J. Timmer, L. C. Sun, Joule 2020, 4, 1408–1444.
- 16A. Tortajada, F. Julia-Hernandez, M. Borjesson, T. Moragas, R. Martin, Angew. Chem. Int. Ed. 2018, 57, 15948–15982; Angew. Chem. 2018, 130, 16178–16214.
- 17M. Aresta, A. Dibenedetto, E. Quaranta, Reaction Mechanisms in Carbon Dioxide Conversion, Springer Berlin, Heidelberg, 2016.
10.1007/978-3-662-46831-9 Google Scholar
- 18N. W. Kinzel, C. Werle, W. Leitner, Angew. Chem. Int. Ed. 2021, 60, 11628–11686; Angew. Chem. 2021, 133, 11732–11792.
- 19
- 19aS. Ren, D. Joulié, D. Salvatore, K. Torbensen, M. Wang, M. Robert, C. P. Berlinguette, Science 2019, 365, 367–369;
- 19bA. Zhanaidarova, S. C. Jones, E. Despagnet-Ayoub, B. R. Pimentel, C. P. Kubiak, J. Am. Chem. Soc. 2019, 141, 17270–17277;
- 19cY. Wu, Z. Jiang, X. Lu, Y. Liang, H. Wang, Nature 2019, 575, 639–642;
- 19dE. Boutin, M. Wang, J. C. Lin, M. Mesnage, D. Mendoza, B. Lassalle-Kaiser, C. Hahn, T. F. Jaramillo, M. Robert, Angew. Chem. Int. Ed. 2019, 58, 16172–16176; Angew. Chem. 2019, 131, 16318–16322;
- 19eZ. Chen, J. Zhang, C. Zhang, R. Cui, M. Tan, S. Guo, H. Wang, J. Jiao, T. Lu, J. Power Sources 2022, 519, 230788;
- 19fM. Wang, L. Chen, T. C. Lau, M. Robert, Angew. Chem. Int. Ed. 2018, 57, 7769–7773; Angew. Chem. 2018, 130, 7895–7899.
- 20
- 20aK. Lei, B. Y. Xia, Chem. Eur. J. 2022, 28, e202200141;
- 20bN. Corbin, J. Zeng, K. Williams, K. Manthiram, Nano Res. 2019, 12, 2093–2125;
- 20cL. Sun, V. Reddu, A. C. Fisher, X. Wang, Energy Environ. Sci. 2020, 13, 374–403;
- 20dM. Robert, C. Costentin, K. Daasbjerg, Carbon Dioxide Electrochemistry Homogeneous and Heterogeneous Catalysis, The Royal Society of Chemistry, London, 2021.
- 21J. Hawecker, J.-M. Lehn, R. Ziessel, J. Chem. Soc. Chem. Commun. 1983, 536–538.
- 22J. Hawecker, J.-M. Lehn, R. Ziessel, J. Chem. Soc. Chem. Commun. 1984, 328–330.
- 23M. Bourrez, F. Molton, S. Chardon-Noblat, A. Deronzier, Angew. Chem. Int. Ed. 2011, 50, 9903–9906; Angew. Chem. 2011, 123, 10077–10080.
- 24B. J. Fisher, R. Eisenberg, J. Am. Chem. Soc. 1980, 102, 7361–7363.
- 25M. Beley, J.-P. Collin, R. Ruppert, J.-P. Sauvage, J. Chem. Soc. Chem. Commun. 1984, 1315–1316.
- 26S. Meshitsuka, M. Ichikawa, K. Tamaru, J. Chem. Soc. Chem. Commun. 1974, 158–159.
- 27C. M. Lieber, N. S. Lewis, J. Am. Chem. Soc. 1984, 106, 5033–5034.
- 28F. Ghani, J. Kristen, H. Riegler, J. Chem. Eng. Data 2012, 57, 439–449.
- 29
- 29aX. Zhang, Y. Wang, M. Gu, M. Wang, Z. Zhang, W. Pan, Z. Jiang, H. Zheng, M. Lucero, H. Wang, G. E. Sterbinsky, Q. Ma, Y.-G. Wang, Z. Feng, J. Li, H. Dai, Y. Liang, Nat. Energy 2020, 5, 684–692;
- 29bX. Zhang, Z. Wu, X. Zhang, L. Li, Y. Li, H. Xu, X. Li, X. Yu, Z. Zhang, Y. Liang, H. Wang, Nat. Commun. 2017, 8, 14675;
- 29cS. Kusama, T. Saito, H. Hashiba, A. Sakai, S. Yotsuhashi, ACS Catal. 2017, 7, 8382–8385;
- 29dF. Lv, N. Han, Y. Qiua, X. Liua, J. Luo, Y. Li, Coord. Chem. Rev. 2020, 422, 213435.
- 30M. Wang, K. Torbensen, D. Salvatore, S. Ren, D. Joulie, F. Dumoulin, D. Mendoza, B. Lassalle-Kaiser, U. Isci, C. P. Berlinguette, M. Robert, Nat. Commun. 2019, 10, 3602.
- 31
- 31aL. Chen, Z. Guo, X. G. Wei, C. Gallenkamp, J. Bonin, E. Anxolabehere-Mallart, K. C. Lau, T. C. Lau, M. Robert, J. Am. Chem. Soc. 2015, 137, 10918–10921;
- 31bZ. Zhang, J. Xiao, X.-J. Chen, S. Yu, L. Yu, R. Si, Y. Wang, S. Wang, X. Meng, Y. Wang, Z.-Q. Tian, D. Deng, Angew. Chem. Int. Ed. 2018, 57, 16339–16342; Angew. Chem. 2018, 130, 16577–16580.
- 32
- 32aK. E. Rivera Cruz, Y. Liu, T. L. Soucy, P. M. Zimmerman, C. C. L. McCrory, ACS Catal. 2021, 11, 13203–13216;
- 32bT. Abe, T. Yoshida, S. Tokita, F. Taguchi, H. Imaya, M. Kaneko, J. Electroanal. Chem. 1996, 412, 125–132.
- 33A. W. Nichols, C. W. Machan, Front. Chem. 2019, 7, 397.
- 34C. G. Zoski, Handbook of Electrochemistry, Elsevier, Oxford, 2007.
- 35C. Costentin, S. Drouet, M. Robert, J. M. Saveant, J. Am. Chem. Soc. 2012, 134, 11235–11242.
- 36
- 36aE. S. Rountree, B. D. McCarthy, T. T. Eisenhart, J. L. Dempsey, Inorg. Chem. 2014, 53, 9983–10002;
- 36bC. Costentin, M. Robert, J. M. Saveant, Chem. Soc. Rev. 2013, 42, 2423–2436;
- 36cC. Costentin, J. M. Saveant, J. Am. Chem. Soc. 2018, 140, 16669–16675.
- 37
- 37aI. Azcarate, C. Costentin, M. Robert, J. M. Saveant, J. Am. Chem. Soc. 2016, 138, 16639–16644;
- 37bI. Azcarate, C. Costentin, M. Robert, J.-M. Savéant, J. Phys. Chem. C 2016, 120, 28951–28960.
- 38M. L. Clark, P. L. Cheung, M. Lessio, E. A. Carter, C. P. Kubiak, ACS Catal. 2018, 8, 2021–2029.
- 39E. E. Benson, K. A. Grice, J. M. Smieja, C. P. Kubiak, Polyhedron 2013, 58, 229–234.
- 40B. P. Sullivan, C. M. Bolinger, D. Conrad, W. J. Vining, T. J. Meyer, J. Chem. Soc. Chem. Commun. 1985, 1414–1416.
- 41E. E. Benson, C. P. Kubiak, Chem. Commun. 2012, 48, 7374–7376.
- 42J. M. Smieja, C. P. Kubiak, Inorg. Chem. 2010, 49, 9283–9289.
- 43Y. Yang, Z. Zhang, Z. Zhang, C. Tang, X. Chang, L. Duan, Chin. J. Chem. 2021, 39, 1281–1287.
- 44D. C. Grills, J. A. Farrington, B. H. Layne, S. V. Lymar, B. A. Mello, J. M. Preses, J. F. Wishart, J. Am. Chem. Soc. 2014, 136, 5563–5566.
- 45M. D. Sampson, A. D. Nguyen, K. A. Grice, C. E. Moore, A. L. Rheingold, C. P. Kubiak, J. Am. Chem. Soc. 2014, 136, 5460–5471.
- 46E. Haviv, D. Azaiza-Dabbah, R. Carmieli, L. Avram, J. M. L. Martin, R. Neumann, J. Am. Chem. Soc. 2018, 140, 12451–12456.
- 47J. H. Jeoung, H. Dobbek, Science 2007, 318, 1461–1464.
- 48M. Beley, J. P. Collin, R. Ruppert, J. P. Sauvage, J. Am. Chem. Soc. 1986, 108, 7461–7467.
- 49J. D. Froehlich, C. P. Kubiak, Inorg. Chem. 2012, 51, 3932–3934.
- 50G. Liu, Y.-J. Fan, J.-L. Zhang, J. Porphyrins Phthalocyanines 2020, 24, 465–472.
- 51K. Talukdar, S. Sinha Roy, E. Amatya, E. A. Sleeper, P. Le Magueres, J. W. Jurss, Inorg. Chem. 2020, 59, 6087–6099.
- 52S. S. Roy, K. Talukdar, J. W. Jurss, ChemSusChem 2021, 14, 662–670.
- 53E. M. Nichols, J. S. Derrick, S. K. Nistanaki, P. T. Smith, C. J. Chang, Chem. Sci. 2018, 9, 2952–2960.
- 54Z. Zhang, P. R. Schreiner, Chem. Soc. Rev. 2009, 38, 1187–1198.
- 55E. M. Nichols, C. J. Chang, Organometallics 2019, 38, 1213–1218.
- 56
- 56aP. Gotico, B. Boitrel, R. Guillot, M. Sircoglou, A. Quaranta, Z. Halime, W. Leibl, A. Aukauloo, Angew. Chem. Int. Ed. 2019, 58, 4504–4509; Angew. Chem. 2019, 131, 4552–4557;
- 56bP. Gotico, L. Roupnel, R. Guillot, M. Sircoglou, W. Leibl, Z. Halime, A. Aukauloo, Angew. Chem. Int. Ed. 2020, 59, 22451–22455; Angew. Chem. 2020, 132, 22637–22641.
- 57
- 57aB. A. Rosen, A. Salehi-Khojin, M. R. Thorson, W. Zhu, D. T. Whipple, P. J. A. Kenis, R. I. Masel, Science 2011, 334, 643–644;
- 57bD. C. Grills, Y. Matsubara, Y. Kuwahara, S. R. Golisz, D. A. Kurtz, B. A. Mello, J. Phys. Chem. Lett. 2014, 5, 2033–2038;
- 57cJ. Medina-Ramos, R. C. Pupillo, T. P. Keane, J. L. DiMeglio, J. Rosenthal, J. Am. Chem. Soc. 2015, 137, 5021–5027;
- 57dJ. Choi, T. M. Benedetti, R. Jalili, A. Walker, G. G. Wallace, D. L. Officer, Chem. Eur. J. 2016, 22, 14158–14161.
- 58S. Sung, D. Kumar, M. Gil-Sepulcre, M. Nippe, J. Am. Chem. Soc. 2017, 139, 13993–13996.
- 59S. Sung, X. Li, L. M. Wolf, J. R. Meeder, N. S. Bhuvanesh, K. A. Grice, J. A. Panetier, M. Nippe, J. Am. Chem. Soc. 2019, 141, 6569–6582.
- 60A. Khadhraoui, P. Gotico, B. Boitrel, W. Leibl, Z. Halime, A. Aukauloo, Chem. Commun. 2018, 54, 11630–11633.
- 61A. Khadhraoui, P. Gotico, W. Leibl, Z. Halime, A. Aukauloo, ChemSusChem 2021, 14, 1308–1315.
- 62C. Costentin, G. Passard, M. Robert, J. M. Saveant, J. Am. Chem. Soc. 2014, 136, 11821–11829.
- 63
- 63aI. Bhugun, D. Lexa, J.-M. Saveant, J. Am. Chem. Soc. 1994, 116, 5015–5016;
- 63bA. Chapovetsky, T. H. Do, R. Haiges, M. K. Takase, S. C. Marinescu, J. Am. Chem. Soc. 2016, 138, 5765–5768.
- 64C. Costentin, S. Drouet, M. Robert, J. M. Saveant, Science 2012, 338, 90–94.
- 65F. Franco, C. Cometto, F. Ferrero Vallana, F. Sordello, E. Priola, C. Minero, C. Nervi, R. Gobetto, Chem. Commun. 2014, 50, 14670–14673.
- 66F. Franco, C. Cometto, L. Nencini, C. Barolo, F. Sordello, C. Minero, J. Fiedler, M. Robert, R. Gobetto, C. Nervi, Chem. Eur. J. 2017, 23, 4782–4793.
- 67Y. Yang, M. Z. Ertem, L. Duan, Chem. Sci. 2021, 12, 4779–4788.
- 68C. G. Margarit, C. Schnedermann, N. G. Asimow, D. G. Nocera, Organometallics 2019, 38, 1219–1223.
- 69K. T. Ngo, M. McKinnon, B. Mahanti, R. Narayanan, D. C. Grills, M. Z. Ertem, J. Rochford, J. Am. Chem. Soc. 2017, 139, 2604–2618.
- 70A. Rana, B. Mondal, P. Sen, S. Dey, A. Dey, Inorg. Chem. 2017, 56, 1783–1793.
- 71P. Sen, B. Mondal, D. Saha, A. Rana, A. Dey, Dalton Trans. 2019, 48, 5965–5977.
- 72
- 72aA. Lashgari, C. K. Williams, J. L. Glover, Y. Wu, J. Chai, J. J. Jiang, Chem. Eur. J. 2020, 26, 16774–16781;
- 72bC. K. Williams, A. Lashgari, J. A. Tomb, J. Chai, J. J. Jiang, ChemCatChem 2020, 12, 4886–4892.
- 73A. Chaturvedi, C. K. Williams, N. Devi, J. J. Jiang, Inorg. Chem. 2021, 60, 3843–3850.
- 74
- 74aC. K. Williams, A. Lashgari, J. Chai, J. J. Jiang, ChemSusChem 2020, 13, 3412–3417;
- 74bN. Devi, C. K. Williams, A. Chaturvedi, J. J. Jiang, ACS Appl. Energy Mater. 2021, 4, 3604–3611.
- 75M. H. Rønne, D. Cho, M. R. Madsen, J. B. Jakobsen, S. Eom, E. Escoude, C. D. Hammershoj, D. U. Nielsen, S. U. Pedersen, M.-H. Baik, T. Skrydstrup, K. Daasbjerg, J. Am. Chem. Soc. 2020, 142, 4265–4275.
- 76S. Amanullah, P. Saha, A. Dey, J. Am. Chem. Soc. 2021, 143, 13579–13592.
- 77C. Costentin, M. Robert, J. M. Saveant, A. Tatin, Proc. Natl. Acad. Sci. USA 2015, 112, 6882–6886.
- 78A. Nakada, O. Ishitani, ACS Catal. 2018, 8, 354–363.
- 79J. J. Walsh, G. Neri, C. L. Smith, A. J. Cowan, Organometallics 2019, 38, 1224–1229.
- 80Y. Wu, G. Hu, C. L. Rooney, G. W. Brudvig, H. Wang, ChemSusChem 2020, 13, 6296–6299.
- 81D. M. Weekes, D. A. Salvatore, A. Reyes, A. Huang, C. P. Berlinguette, Acc. Chem. Res. 2018, 51, 910–918.
- 82N. Sonoyama, M. Kirii, T. Sakata, Electrochem. Commun. 1999, 1, 213–216.
- 83D.-D. Ma, S.-G. Han, C. Cao, X. Li, X.-T. Wu, Q.-L. Zhu, Appl. Catal. B 2020, 264, 118530.
- 84X. M. Hu, M. H. Ronne, S. U. Pedersen, T. Skrydstrup, K. Daasbjerg, Angew. Chem. Int. Ed. 2017, 56, 6468–6472; Angew. Chem. 2017, 129, 6568–6572.
- 85J. Choi, P. Wagner, S. Gambhir, R. Jalili, D. R. MacFarlane, G. G. Wallace, D. L. Officer, ACS Energy Lett. 2019, 4, 666–672.
- 86J. D. Blakemore, A. Gupta, J. J. Warren, B. S. Brunschwig, H. B. Gray, J. Am. Chem. Soc. 2013, 135, 18288–18291.
- 87
- 87aA. Maurin, M. Robert, J. Am. Chem. Soc. 2016, 138, 2492–2495;
- 87bB. Reuillard, K. H. Ly, T. E. Rosser, M. F. Kuehnel, I. Zebger, E. Reisner, J. Am. Chem. Soc. 2017, 139, 14425–14435;
- 87cS. Pugliese, N. T. Huan, J. Forte, D. Grammatico, S. Zanna, B. L. Su, Y. Li, M. Fontecave, ChemSusChem 2020, 13, 6449–6456.
- 88J. Choi, P. Wagner, R. Jalili, J. Kim, D. R. MacFarlane, G. G. Wallace, D. L. Officer, Adv. Energy Mater. 2018, 8, 1801280.
- 89M. Zhu, C. Cao, J. Chen, Y. Sun, R. Ye, J. Xu, Y.-F. Han, ACS Appl. Energy Mater. 2019, 2, 2435–2440.
- 90K. Torbensen, C. Han, B. Boudy, N. von Wolff, C. Bertail, W. Braun, M. Robert, Chem. Eur. J. 2020, 26, 3034–3038.
- 91
- 91aC. Floriani, G. Fachinetti, J. Chem. Soc. Chem. Commun. 1974, 615–616;
- 91bM. Zhu, J. Chen, R. Guo, J. Xu, X. Fang, Y.-F. Han, Appl. Catal. B 2019, 251, 112–118;
- 91cW. W. Kramer, C. C. L. McCrory, Chem. Sci. 2016, 7, 2506–2515.
- 92T. Atoguchi, A. Aramata, A. Kazusaka, M. Enyo, J. Chem. Soc. Chem. Commun. 1991, 156–157.
- 93M. Zhu, J. Chen, L. Huang, R. Ye, J. Xu, Y. F. Han, Angew. Chem. Int. Ed. 2019, 58, 6595–6599; Angew. Chem. 2019, 131, 6667–6671.
- 94S. A. Yao, R. E. Ruther, L. Zhang, R. A. Franking, R. J. Hamers, J. F. Berry, J. Am. Chem. Soc. 2012, 134, 15632–15635.
- 95A. Maurin, M. Robert, Chem. Commun. 2016, 52, 12084–12087.
- 96A. N. Marianov, Y. Jiang, Appl. Catal. B 2019, 244, 881–888.
- 97J. Su, J.-J. Zhang, J. Chen, Y. Song, L. Huang, M. Zhu, B. I. Yakobson, B. Z. Tang, R. Ye, Energy Environ. Sci. 2021, 14, 483–492.
- 98M. N. Jackson, Y. Surendranath, Acc. Chem. Res. 2019, 52, 3432–3441.
- 99C. J. Kaminsky, J. Wright, Y. Surendranath, ACS Catal. 2019, 9, 3667–3671.
- 100S. Oh, J. R. Gallagher, J. T. Miller, Y. Surendranath, J. Am. Chem. Soc. 2016, 138, 1820–1823.
- 101M. N. Jackson, S. Oh, C. J. Kaminsky, S. B. Chu, G. Zhang, J. T. Miller, Y. Surendranath, J. Am. Chem. Soc. 2018, 140, 1004–1010.
- 102K. L. Materna, R. H. Crabtree, G. W. Brudvig, Chem. Soc. Rev. 2017, 46, 6099–6110.
- 103S. Roy, M. Miller, J. Warnan, J. J. Leung, C. D. Sahm, E. Reisner, ACS Catal. 2021, 11, 1868–1876.
- 104B. L. Wadsworth, D. Khusnutdinova, G. F. Moore, J. Mater. Chem. A 2018, 6, 21654–21665.
- 105S. Sato, B. J. McNicholas, R. H. Grubbs, Chem. Commun. 2020, 56, 4440–4443.
- 106W.-S. Huang, B. D. Humphrey, A. G. MacDiarmid, J. Chem. Soc. Faraday Trans. 1 1986, 82, 2385–2400.
- 107
- 107aE. Portenkirchner, K. Oppelt, C. Ulbricht, D. A. M. Egbe, H. Neugebauer, G. Knör, N. S. Sariciftci, J. Organomet. Chem. 2012, 716, 19–25;
- 107bE. Portenkirchner, J. Gasiorowski, K. Oppelt, S. Schlager, C. Schwarzinger, H. Neugebauer, G. Knor, N. S. Sariciftci, ChemCatChem 2013, 5, 1790–1796.
- 108D. H. Apaydin, E. Tordin, E. Portenkirchner, G. Aufischer, S. Schlager, M. Weichselbaumer, K. Oppelt, N. S. Sariciftci, ChemistrySelect 2016, 1, 1156–1162.
- 109C. Sun, S. Prosperini, P. Quagliotto, G. Viscardi, S. S. Yoon, R. Gobetto, C. Nervi, Dalton Trans. 2016, 45, 14678–14688.
- 110J. Willkomm, S. Bouzidi, E. Bertin, V. I. Birss, W. E. Piers, ACS Catal. 2021, 11, 1096–1105.
- 111X. M. Hu, Z. Salmi, M. Lillethorup, E. B. Pedersen, M. Robert, S. U. Pedersen, T. Skrydstrup, K. Daasbjerg, Chem. Commun. 2016, 52, 5864–5867.
- 112N. G. Yasri, T. A. Al-Attas, J. Hu, M. G. Kibria, Catal. Sci. Technol. 2021, 11, 1580–1589.
- 113
- 113aN. Han, Y. Wang, L. Ma, J. Wen, J. Li, H. Zheng, K. Nie, X. Wang, F. Zhao, Y. Li, J. Fan, J. Zhong, T. Wu, D. J. Miller, J. Lu, S.-T. Lee, Y. Li, Chem 2017, 3, 652–664;
- 113bJ. Chen, J. Li, W. Liu, X. Ma, J. Xu, M. Zhu, Y.-F. Han, Green Chem. 2019, 21, 6056–6061.
- 114R.-B. Lin, B. Chen, Joule 2018, 2, 1030–1032.
- 115A. Bavykina, N. Kolobov, I. S. Khan, J. A. Bau, A. Ramirez, J. Gascon, Chem. Rev. 2020, 120, 8468–8535.
- 116N. Kornienko, Y. Zhao, C. S. Kley, C. Zhu, D. Kim, S. Lin, C. J. Chang, O. M. Yaghi, P. Yang, J. Am. Chem. Soc. 2015, 137, 14129–14135.
- 117I. Hod, M. D. Sampson, P. Deria, C. P. Kubiak, O. K. Farha, J. T. Hupp, ACS Catal. 2015, 5, 6302–6309.
- 118Z. Meng, J. Luo, W. Li, K. A. Mirica, J. Am. Chem. Soc. 2020, 142, 21656–21669.
- 119
- 119aL. Sun, M. G. Campbell, M. Dinca, Angew. Chem. Int. Ed. 2016, 55, 3566–3579; Angew. Chem. 2016, 128, 3628–3642;
- 119bL. S. Xie, G. Skorupskii, M. Dinca, Chem. Rev. 2020, 120, 8536–8580.
- 120R. Matheu, E. Gutierrez-Puebla, M. A. Monge, C. S. Diercks, J. Kang, M. S. Prevot, X. Pei, N. Hanikel, B. Zhang, P. Yang, O. M. Yaghi, J. Am. Chem. Soc. 2019, 141, 17081–17085.
- 121M. Chen, H. Li, C. Liu, J. Liu, Y. Feng, A. G. H. Wee, B. Zhang, Coord. Chem. Rev. 2021, 435, 213778.
- 122S. Lin, C. S. Diercks, Y. B. Zhang, N. Kornienko, E. M. Nichols, Y. Zhao, A. R. Paris, D. Kim, P. Yang, O. M. Yaghi, C. J. Chang, Science 2015, 349, 1208–1213.
- 123H. Liu, J. Chu, Z. Yin, X. Cai, L. Zhuang, H. Deng, Chem 2018, 4, 1696–1709.
- 124B. Han, X. Ding, B. Yu, H. Wu, W. Zhou, W. Liu, C. Wei, B. Chen, D. Qi, H. Wang, K. Wang, Y. Chen, B. Chen, J. Jiang, J. Am. Chem. Soc. 2021, 143, 7104–7113.
- 125C. S. Diercks, S. Lin, N. Kornienko, E. A. Kapustin, E. M. Nichols, C. Zhu, Y. Zhao, C. J. Chang, O. M. Yaghi, J. Am. Chem. Soc. 2018, 140, 1116–1122.
- 126Y. Lu, J. Zhang, W. Wei, D. D. Ma, X. T. Wu, Q. L. Zhu, ACS Appl. Mater. Interfaces 2020, 12, 37986–37992.
- 127
- 127aH. Q. Liang, T. Beweries, R. Francke, M. Beller, Angew. Chem. Int. Ed. 2022, 61, e202200723; Angew. Chem. 2022, 134, e202200723;
- 127bE. Boutin, M. Robert, Trends Chem. 2021, 3, 359–372.
- 128S. Zhao, Y. Yang, Z. Tang, Angew. Chem. Int. Ed. 2022, 61, e202110186; Angew. Chem. 2022, 134, e202110186.
- 129Z. Weng, Y. Wu, M. Wang, J. Jiang, K. Yang, S. Huo, X. F. Wang, Q. Ma, G. W. Brudvig, V. S. Batista, Y. Liang, Z. Feng, H. Wang, Nat. Commun. 2018, 9, 415.
- 130N. Nandal, S. L. Jain, Coord. Chem. Rev. 2022, 451, 214271.
- 131L.-W. Chen, H.-W. Liang, Catal. Sci. Technol. 2021, 11, 4673–4689.
- 132S. Verma, S. Lu, P. J. A. Kenis, Nat. Energy 2019, 4, 466–474.