Highly Selective Carbonylation of CH3Cl to Acetic Acid Catalyzed by Pyridine-Treated MOR Zeolite
Xudong Fang
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorDr. Fuli Wen
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorXiangnong Ding
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorHanbang Liu
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorDr. Zhiyang Chen
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorZhaopeng Liu
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Dr. Hongchao Liu
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Wenliang Zhu
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
Search for more papers by this authorProf. Zhongmin Liu
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorXudong Fang
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorDr. Fuli Wen
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorXiangnong Ding
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorHanbang Liu
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorDr. Zhiyang Chen
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorZhaopeng Liu
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Dr. Hongchao Liu
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Wenliang Zhu
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
Search for more papers by this authorProf. Zhongmin Liu
National Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorGraphical Abstract
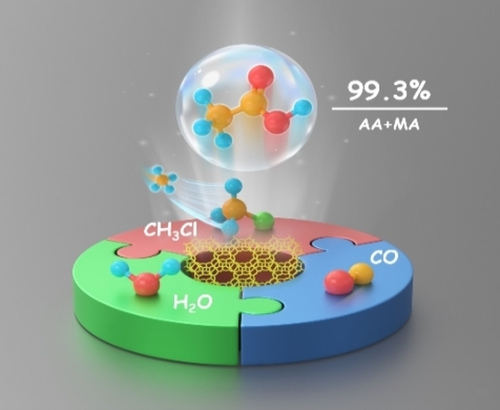
An innovative reaction strategy for the transformation of methane into acetic acid via CH3Cl carbonylation over H-zeolites is reported. A 99.3 % acetic acid (AA) and methyl acetate (MA) selectivity was obtained over pyridine-pretreated MOR at 523 K and 2.0 MPa. This strategy enables efficient conversion of methane into oxygenates under mild conditions.
Abstract
The selective conversion of methane to high value-added chemicals under mild conditions is of great significance for the commercially viable and sustainable utilization of methane but remains a formidable challenge. Herein, we report a strategy for efficiently converting methane to acetic acid via CH3Cl as an intermediate. Up to 99.3 % acetic acid and methyl acetate (AA+MA) selectivity was achieved over pyridine-pretreated MOR (MOR-8) under moderate conditions of 523 K and 2.0 MPa. Water, conventionally detrimental to carbonylation reaction over zeolite catalysts, was conducive to the production of AA in the current reaction system. In the 100 h continuous test with the MOR-8 catalyst, the average AA+MA selectivity remained over 98 %. AA was formed by carbonylation of methoxy groups within 8-membered rings of MOR followed by hydrolysis. This strategy provided an approach for highly efficient utilization of methane to oxygenates under mild reaction conditions.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202203859-sup-0001-misc_information.pdf2.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. Huang, J. B. Miller, G. W. Huber, J. A. Dumesic, C. T. Maravelias, Joule 2018, 2, 349–365;
- 1bX. Meng, X. Cui, N. P. Rajan, L. Yu, D. Deng, X. Bao, Chem 2019, 5, 2296–2325.
- 2
- 2aX. Yu, V. L. Zholobenko, S. Moldovan, D. Hu, D. Wu, V. V. Ordomsky, A. Y. Khodakov, Nat. Energy 2020, 5, 511–519;
- 2bA. I. Olivos-Suarez, A. Szecsenyi, E. J. M. Hensen, J. Ruiz-Martinez, E. A. Pidko, J. Gascon, ACS Catal. 2016, 6, 2965–2981.
- 3
- 3aY. Wang, Joule 2018, 2, 1399–1401;
- 3bP. Schwach, X. Pan, X. Bao, Chem. Rev. 2017, 117, 8497–8520.
- 4
- 4aZ. Jin, L. Wang, E. Zuidema, K. Mondal, M. Zhang, J. Zhang, C. T. Wang, X. J. Meng, H. Q. Yang, C. Mesters, F. S. Xiao, Science 2020, 367, 193–197;
- 4bJ. F. Wu, S. M. Yu, W. D. Wang, Y. X. Fan, S. Bai, C. W. Zhang, Q. Gao, J. Huang, W. Wang, J. Am. Chem. Soc. 2013, 135, 13567–13573;
- 4cW. Huang, K. C. Xie, J. P. Wang, Z. H. Gao, L. H. Yin, Q. M. Zhu, J. Catal. 2001, 201, 100–104.
- 5
- 5aP. Tang, Q. Zhu, Z. Wu, D. Ma, Energy Environ. Sci. 2014, 7, 2580–2591;
- 5bY. Liu, D. Deng, X. Bao, Chem 2020, 6, 2497–2514.
- 6R. Lin, A. P. Amrute, J. Perez-Ramirez, Chem. Rev. 2017, 117, 4182–4247.
- 7C. Tu, X. Nie, J. G. Chen, ACS Catal. 2021, 11, 3384–3401.
- 8
- 8aR. A. Periana, O. Mironov, D. Taube, G. Bhalla, C. J. Jones, Science 2003, 301, 814–818;
- 8bJ. Shan, M. Li, L. F. Allard, S. Lee, M. Flytzani-Stephanopoulos, Nature 2017, 551, 605–608.
- 9K. Narsimhan, V. K. Michaelis, G. Mathies, W. R. Gunther, R. G. Griffin, Y. Roman-Leshkov, J. Am. Chem. Soc. 2015, 137, 1825–1832.
- 10Y. Tang, Y. Li, V. Fung, D. E. Jiang, W. Huang, S. Zhang, Y. Iwasawa, T. Sakata, L. Nguyen, X. Zhang, A. I. Frenkel, F. F. Tao, Nat. Commun. 2018, 9, 1231.
- 11
- 11aG. Zichittella, V. Paunović, A. P. Amrute, J. Pérez-Ramírez, ACS Catal. 2017, 7, 1805–1817;
- 11bM. Bilke, P. Losch, O. Vozniuk, A. Bodach, F. Schuth, J. Am. Chem. Soc. 2019, 141, 11212–11218.
- 12Y. X. Wei, D. Z. Zhang, Z. M. Liu, B. L. Su, J. Catal. 2006, 238, 46–57.
- 13P. Wang, L. Chen, J.-K. Guo, S. Shen, C.-T. Au, S.-F. Yin, Ind. Eng. Chem. Res. 2019, 58, 18582–18589.
- 14X. Fang, H. Liu, Z. Chen, Z. Liu, X. Ding, Y. Ni, W. Zhu, Z. Liu, Angew. Chem. Int. Ed. 2022, 61, e202114953; Angew. Chem. 2022, 134, e202114953.
- 15
- 15aK. X. Wang, H. F. Xu, W. S. Li, C. T. Au, X. P. Zhou, Appl. Catal. A 2006, 304, 168–177;
- 15bY. F. Fan, D. Ma, X. H. Bao, Catal. Lett. 2009, 130, 286–290.
- 16T. Shikada, H. Yagita, K. Fujimoto, H. Tominaga, Appl. Catal. 1986, 22, 379–384.
- 17M. Guisnet, P. Magnoux, Appl. Catal. 1989, 54, 1–27.
- 18J. Qi, J. Finzel, H. Robatjazi, M. Xu, A. S. Hoffman, S. R. Bare, X. Pan, P. Christopher, J. Am. Chem. Soc. 2020, 142, 14178–14189.
- 19
- 19aZ. Chen, Y. Ni, Y. Zhi, F. Wen, Z. Zhou, Y. Wei, W. Zhu, Z. Liu, Angew. Chem. Int. Ed. 2018, 57, 12549–12553; Angew. Chem. 2018, 130, 12729–12733;
- 19bF. Wen, J. Zhang, Z. Chen, Z. Zhou, H. Liu, W. Zhu, Z. Liu, Catal. Sci. Technol. 2021, 11, 1358–1364;
- 19cC. Wei, Q. Yu, J. Li, Z. Liu, ACS Catal. 2020, 10, 4171–4180;
- 19dC. Wei, J. Li, K. Yang, Q. Yu, S. Zeng, Z. Liu, Chem Catal. 2021, 1, 1273–1290.
- 20
- 20aP. Cheung, A. Bhan, G. J. Sunley, E. Iglesia, Angew. Chem. Int. Ed. 2006, 45, 1617–1620; Angew. Chem. 2006, 118, 1647–1650;
- 20bK. De Wispelaere, C. S. Wondergem, B. Ensing, K. Hemelsoet, E. J. Meijer, B. M. Weckhuysen, V. Van Speybroeck, J. Ruiz-Martínez, ACS Catal. 2016, 6, 1991–2002.
- 21Q. Zhang, J. Yu, A. Corma, Adv. Mater. 2020, 32, 2002927.
- 22B. Lu, T. Kanai, Y. Oumi, T. Sano, J. Porous Mater. 2007, 14, 89–96.
- 23D. K. Murray, T. Howard, P. W. Goguen, T. R. Krawietz, J. F. Haw, J. Am. Chem. Soc. 1994, 116, 6354–6360.