From Copper to Basic Copper Carbonate: A Reversible Conversion Cathode in Aqueous Anion Batteries
Graphical Abstract
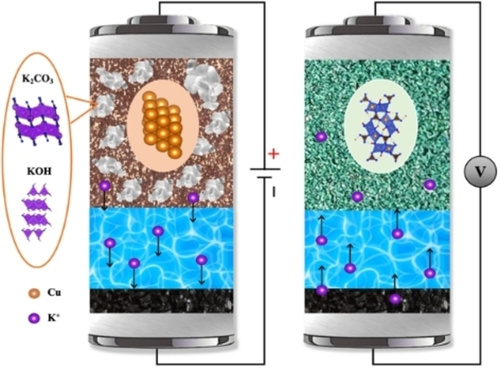
The highly reversible electrochemical conversion of commercial bulk copper metal to form basic copper carbonate, Cu2CO3(OH)2, from a saturated aqueous electrolyte comprising 4.5 m K2CO3 and 9 m KOH, demonstrating high capacity, fast rate capability, and relatively stable cycle life is reported. The salt electrode consisting of Cu, K2CO3, and KOH renders this anion-hosting electrode a cathode for rocking-chair batteries.
Abstract
Dual-ion batteries that use anions and cations as charge carriers represent a promising energy-storage technology. However, an uncharted area is to explore transition metals as electrodes to host carbonate in conversion reactions. Here we report the reversible conversion reaction from copper to Cu2CO3(OH)2, where the copper electrode comprising K2CO3 and KOH solid is self-sufficient with anion-charge carriers. This electrode dissociates and associates K+ ions during battery charge and discharge. The copper active mass and the anion-bearing cathode exhibit a reversible capacity of 664 mAh g−1 and 299 mAh g−1, respectively, and relatively stable cycling in a saturated mixture electrolyte of K2CO3 and KOH. The results open an avenue to use carbonate as a charge carrier for batteries to serve for the consumption and storage of CO2.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.