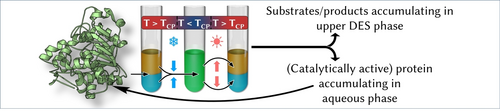
A Deep Eutectic Solvent Thermomorphic Multiphasic System for Biocatalytic Applications
Graphical Abstract
The applicability of a deep eutectic solvent thermomorphic multiphasic system (DES-TMS) within biocatalysis was successfully demonstrated. Horse liver alcohol dehydrogenase was simply recycled using the DES-TMS. At increased temperatures, the system splits into an aqueous-enriched and a DES-enriched phase. In this way, the product and the catalyst were separated in a single unit operation step.
Abstract
The applicability of a thermomorphic multiphasic system (TMS) composed of a hydrophobic deep eutectic solvent (DES) and an aqueous potassium phosphate buffer with a lower critical solution temperature (LCST) phase change for homogeneous biocatalysis was investigated. A lidocaine-based DES with the fatty acid oleic acid as a hydrogen-bond donor was studied. Phase diagrams were determined and presented within this study. We tested different additional components to the solvent system and observed a decrease in the cloud point of approximately 0.026 °C per concentration unit. Distribution studies revealed a clear distribution of the protein in the aqueous buffer phase (>95 %), whereas the hydrophobic substrate and educt accumulated (>95 %) in the DES-enriched layer. Finally, a reduction catalyzed by horse liver alcohol dehydrogenase was performed in a larger-scale experiment, and the biocatalyst could be recycled by simply removing the DES phase for three recycling runs.
Introduction
Only nine out of 51 routinely used solvents were classified as “recommended” based on a survey of global companies, such as Pfizer, Astra Zeneca, GSK, and Sanofi, and working groups, such as the ACS Green Chemistry Institute Pharmaceutical Roundtable (GCI-PR).1 Organic solvents are ubiquitous in laboratories for organic synthesis and for industrial synthetic chemistry. Unfortunately, their use suffers from inherent toxicity and high volatility resulting in the release of harmful organic compounds into the environment. In addition, safety considerations must be well thought-out when working with organic solvents due to their flammability and immediate impact on laboratory personnel through inhalation and resorption. In the last decades, novel classes of “greener”, safer and more sustainable solvents were developed and extensively studied,2 for example, “molten salts” ionic liquids (ILs),3 supercritical fluids (SCFs),4 and deep eutectic solvents (DESs).5
Biotechnology, on the other hand, additionally addresses current and upcoming demands of our society for the sustainable industrial production of chemicals as powerful substitutes for established chemical processes.6 This success story is proven by major industry examples of bio-based bulk chemicals, such as (bio)ethanol, antibiotics, acrylamide and various intermediates for active pharmaceutical ingredients (APIs).7 Today, several hundreds of industrial biocatalytic processes are applied, and we will witness that the number will rise progressively in the future.8 Enzymes generally benefit from i) exclusive chemo-, stereo- and/or enantioselectivity, ii) mild reaction conditions, iii) a broad substrate spectrum and iv) their low intrinsic environmental impact, as enzymes are considered to be fully biodegradable.9 Due to their cellular origin, enzymes operate best in water-enriched environments. However, biocatalysts lose activity (except some examples of hydrolases) when water is replaced with alternative organic solvents. Additionally, many synthetically interesting substrates are not soluble in aqueous (AQ) media and therefore, significantly lower productivities are observed. Consequently, researchers began to use nonconventional media in biocatalysis for higher enzymatic activities and higher solubility of substrates,10 and in this context DESs can be the solution for overcoming these limitations.11
DESs, being knighted as “solvents for the 21st Century”,12 or as “the organic reaction medium of the century”,13 impress with their ecological, economical and practical advantages. First introduced in a pioneering work by Abbott et al. in 2003,14 DESs have been used in many research fields,15 and have recently also found their way into biocatalysis.16 In brief, many DESs are based on their biogenic origin (e.g. choline chloride, carboxylic acids, urea, citric acid, succinic acid, and glycerol) entailing i) melting points below room temperature, ii) high thermal stability, iii) low volatility, iv) diminished toxicity, v) better biodegradability, vi) easier preparation methods, vii) large availability at acceptable costs and viii) tailored solvent characteristics. Overall, DESs have a low ecological footprint and although many of these properties are also transferable to ILs, the inherent biodegradability, biocompatibility, and sustainability of DESs make them a promising alternative to ILs in some applications—but certainly not in all cases and far from entirety.
Generally, a DES is composed of a hydrogen-bond donor (HBD), such as urea, citric acid or sorbitol, and a hydrogen-bond acceptor (HBA), such as chlorine chloride, glycine, or lactic acid.17 From this incomplete list, the hidden potential becomes clear: by combining the various components in different proportions, a new liquid phase by self-association is formed with a significantly lower freezing point than that of the individual substances. According to the nature of the DESs’ properties, they are fully, partly, or not at all miscible with hydrophilic solvents like water or aqueous buffer solutions. Hydrophobic DESs were just very recently studied, and we would like to highlight the brilliantly written review article from van Osch et al. in this context.18 In 2011, Bica et al. first reported on anesthetic lidocaine-based hydrophobic DESs.19 Nine years later, Longeras et al. reported an abrupt phase separation while increasing the temperature when lidocaine-based hydrophobic DES-water mixtures were used.25 Systems with a temperature-dependent phase change are aqueous two-phase (ATPS) thermomorphic multiphasic systems (TMSs) and were already reported in the literature. Briefly, a combination of different fractions of components such as buffer salts, water, and hydrophobic substances (e.g., ILs, polymers, organic solvents) form differently shaped miscibility gaps causing a macroscopic phase change from monophasic to biphasic or vice versa.20 Most commonly, miscibility gaps with an upper or lower critical solution temperature (UCST or LCST, respectively) are described in the literature. Beneficially, a reaction being performed at monophasic conditions is not restricted by mass transfer limitations and can therefore exploit its full potential with the desired solubility of substrates. Whereas two-phasic conditions are beneficial for the downstream processing which can be tremendously improved since often the biocatalyst and the product distribute significantly different into the two phases upon the temperature change.21 Herein, we highlight a recent review from Bianga et al. for elaborately analyzing TMSs.22
In 2011, Behr et al. published a liquid immobilization concept for enzymes by TMSs.23 Here, a ternary mixture of water, hexanol, and methanol was used for the lipase-catalyzed hydrolysis of para-nitrophenyl palmitate. Amano lipase PS from Burkholderia cepacia was used as the biocatalyst and only a minor loss (2 %) in product yields over five sequential recycling runs was reported. Recently, Langermann and co-workers described IL-water-based TMSs for biocatalytic reactions with UCST phase behavior for homogeneous Candida antarctica lipase B (CalB)-catalyzed kinetic resolution of (rac)-1-phenylethyl acetate.24 A high enzymatic activity and full conversion within the IL-based TMSs were observed and the biocatalyst was easily recycled after phase separation at lower temperatures and six consecutive reaction cycles were performed.
In this publication, we present our findings of a LCST-DES-TMS for the integration of a reaction–extraction downstream processing approach (Figure 1). To the best of our knowledge, the use of a thermomorphic DES in biocatalytic applications is the first time it has been reported in the literature. By the combination of a hydrophobic DES and an aqueous (phosphate buffer) phase, a temperature-controlled multicomponent solvent system is formed which switches between biphasic at higher and monophasic at lower temperatures. We report on a simple (catalytically active) protein liquid-immobilization within the aqueous phase combined with a product stream pre-concentration in one unit operation. In addition, an enzyme-catalyzed reaction is carried out at lower temperatures in the monophasic system, and afterwards, the biocatalyst and reactants/products can be separated easily at biphasic conditions only by slightly increasing the temperature. In all cases, the enzyme remains mostly in the aqueous phase whereas the hydrophobic products and unreacted educts stay in the likewise hydrophobic DES phase. We believe that our process intensification idea solves several industrial challenges: i) reactions occur at low temperatures, which is beneficial for energy-efficient synthesis, and for sensitive biocatalysts and organic components, ii) downstream processing is simplified by selective extraction of biocatalysts and educts/products in one unit operation, for example, for the isolation of bulk chemicals from fermentative processes, and/or for the separation of the involved biocatalysts from the reaction broth, as well as iii) recovery and reuse of the biocatalysts (enzymes or whole cells) through liquid-immobilization.
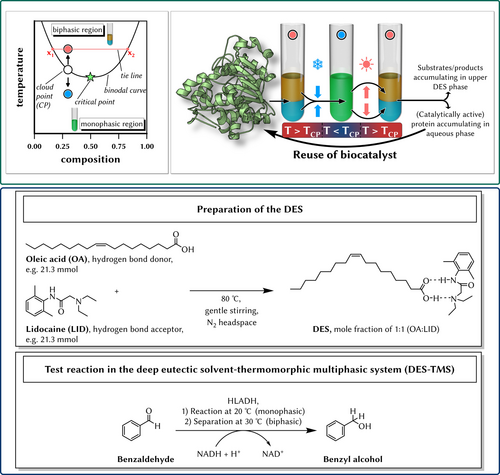
Hypothetical phase diagram illustrating a multicomponent system with a lower critical solution temperature (LCST) miscibility gab (left, green box), and the concept of this work of the LCST thermo-regulated multicomponent deep eutectic solvent system (LCST-DES-TMS) for either the extraction of proteins and/or a biocatalyzed reaction. Together with a follow-up extraction step in one unit operation step, the downstream processing is significantly simplified (right, green box). The DES used in this study was prepared by mixing oleic acid and lidocaine in the molar ratio of 1 : 1 (preparation of the DES, blue box). As a test reaction, the horse liver alcohol dehydrogenase (HLADH)-catalyzed reduction of benzaldehyde to benzyl alcohol was investigated (test rection in the TMS-DES, blue box).
Results and Discussion
Initially, we investigated the behavior of the two components of the DES. Different fractions of lidocaine and oleic acid showed significantly lower melting temperatures compared to the individual components (data not shown). These results are in excellent agreement with the literature, where the eutectic point has also been estimated to be −60 °C.19, 25
To gain initial insights into the thermomorphic behavior of the DES, the phase behavior with ultrapure water (UPW) was first examined in more detail and the respective binodal curves were determined (Figure 2). We found a LCST critical point of 25.5±0.5 °C at wDES=50 %. This result is in very good correlation with the value reported by Longeras et al. (TLongeras et al.=25 °C).25 Overall, a “U”-shaped binodal was yielded and the phenomenon of LCST phase behavior can be explained by focusing on the hydrogen bonds between the components: At low temperatures, strong hydrogen bonds form within all species, but these break after the temperature increases due to the intensification of molecular rotation. A LCST system then splits into two phases when the London dispersion force interactions between like species are stronger than those between unlike species.26 Lidocaine, as an amphiphilic amine being the hydrogen-bond acceptor in the investigated DES, has a temperature dependent pKa value of 8 underlining the LCST phase behavior of the systems.27
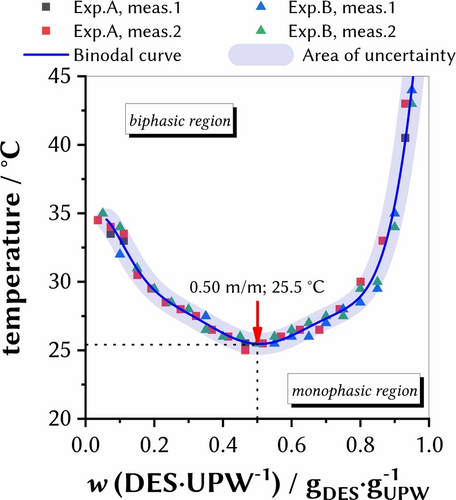
LCST-phase diagram of lidocaine-based DES with UPW as the second component. The blue straight binodal curve is a guide for the eye without any mathematical relationship, and the area of uncertainty represents the device temperature accuracy.
After our initial experiments, we investigated the effects of buffer salts on the cloud point temperatures of the DES system. Although enzymes need water to operate, it is well known that the application of buffer salts significantly increase the stability of enzymes due to a controlled pH value and a controlled ionic strength of the solution cohering the enzyme's quaternary structure. Surprisingly, we observed a noteworthy differently “W”-shaped binodal curve if potassium phosphate buffer solutions (KPi buffer, different concentrations, and different pH values) were combined with the DES (Figure 3). Investigating KPi buffers with pH 7.5 in different concentrations, we observed that the LCST cloud-point slightly decreased from 25.5±0.5 °C (for wDES=50 % and UPW) to 23.5±0.5 °C (for wDES=50 % and 500 mmol L−1 KPi buffer, pH 7.5, Figure 3, left). Second, we observed the appearance of two local minima with two critical points the higher the KPi buffer concentrations are, for example, for 500 mmol L−1 KPi buffer (pH 7.5), a first minimum at 22.0±0.5 °C for wDES=20 % and a second minimum at 23.0±0.5 °C for wDES=75 % (Figure 3, left). Investigating KPi buffers with pH 6.0 in different concentrations, we unexpectedly measured binodal curves with an inverse shape at wDES=0 % to 50 % (Figure 3, right). However, the trend of the previous findings was continued: The higher the concentration of the buffer salts, the lower the binodal of the respective system. When comparing the different pH values at the same fraction of wDES=50 %, we found lower cloud point temperatures for each concentration, 25.5±0.5 °C (pH 7.5) versus 23.0±0.5 °C (pH 6.0) for 50 mmol L−1 KPi buffer and 23.5±0.5 °C (pH 7.5) versus 22.5±0.5 °C (pH 6.0) for 100 mmol L−1 KPi buffer. To the best of our knowledge, this is the first observation on the influence of potassium phosphate buffer salts towards the binodal of DES/KPi buffer mixtures with LCST phase behavior.
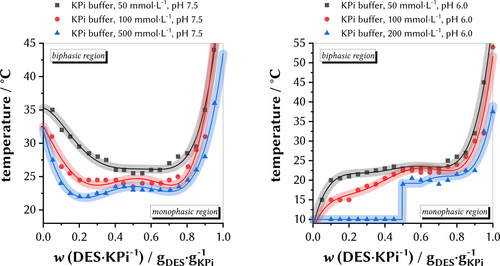
LCST phase diagrams of DES and different phosphate buffer (KPi) concentrations as the second component at pH 7.5 (left) and pH 6.0 (right). The straight binodal curves are a guide for the eyes without any mathematical relationship and the area of uncertainty represents the devices temperature accuracy. For 200 mmol L−1 KPi, pH 6 (right), the first eight measurements were below the cooling capabilities of the device and were therefore set to 10 °C.
As a next step, we investigated the influence of additional substances to the final reaction system. The experiments aimed to rigorously mimicking a large-scale recycling experiment. The addition of either benzaldehyde (substrate), benzyl alcohol (product), and NADH (cofactor) results in a decrease of the cloud point of the system composed of 50 mmol L−1 KPi (pH 6.0) and a mass fraction of wDES=50 % (Figure 4, left). A decrease of approximately 0.026 °C per concentration unit was observed for all compounds. However, if moderate concentrations of substrate and cofactor of about 10–50 mM are applied, the effect is not significant (ca. 0.26 °C to 1.3 °C of a decreased critical temperature, respectively). We choose BSA as a model protein and fortunately, no substantial change of the cloud-point temperature was observed in a typically applied protein concentration in a system composed of 100 mmol L−1 KPi (pH 7.5) with a mass fraction of wDES=50 %. The same result was found, if the enzyme HLADH was added to a system composed of 50 mmol L−1 KPi (pH 6.0) with a mass fraction of wDES=50 % (Figure 4, right). Distribution studies were carried out to investigate the phase into which the protein and products/educts being extracted. First, we studied the extraction of proteins into the upper, DES-enriched phase, and into the lower, AQ-enriched phase. Therefore, 800 mg L−1 bovine serum albumin (BSA) were dissolved in 50 mmol L−1 KPi (pH 6.0) and wDES=50 % at lower temperatures (monophasic conditions). Afterwards, phase separation was promoted, and individual samples were taken from both phases which were then analyzed with SDS polyacrylamide gel electrophoresis (Figure 5). A blank sample without protein indicated, as expected, no bands in either the DES- or AQ-enriched phase between 55–70 kDa (Figure 5, lane 5/6). In contrast, a system with BSA clearly revealed the distribution of the model protein within the AQ-enriched phase (Figure 5, lane 8). Only a small protein band was observed for the DES-enriched phase (Figure 5, lane 9). We estimate an extraction of BSA into the AQ-enriched layer of >95 %. This finding impressively shows that the protein predominantly accumulates in the AQ-enriched phase of the DES-TMS system.
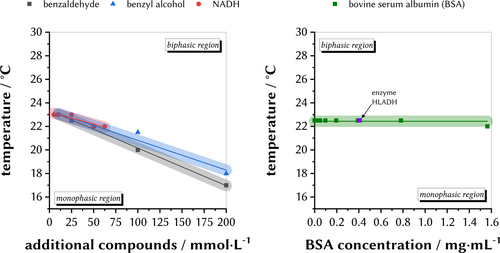
Influence of the substrate benzaldehyde, the product benzyl alcohol, and the cofactor NADH in a system composed of 50 mmol L−1 KPi (pH 6.0) and a mass fraction of wDES=50 % (left). Influence of the model protein BSA towards the cloud-point temperature of a system composed of 100 mmol L−1 KPi (pH 7.5) and a mass fraction of wDES=50 % (right). The light area around the curves represents the device temperature accuracy.
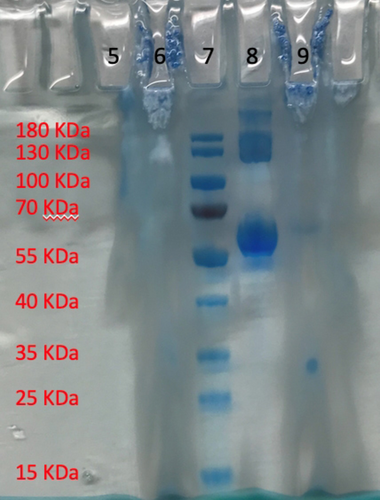
SDS-gel of the distribution of protein BSA in the upper DES-enriched phase and lower AQ-enriched buffer layer with 800 mg mL−1 BSA. A system of 50 mmol L−1 KPi (pH 6.0) and a mass fraction of wDES=50 % was used. Blank AQ-enriched phase (5), blank DES-phase (6), PageRulerTM prestained protein ladder (7). BSA in the AQ-enriched phase (8), and in the DES-enriched phase after separating the phases at temperatures above the cloud-point temperature (9).
In the next step, we studied the distribution of educts and products within a system composed of 50 mmol L−1 KPi (pH 6.0) and wDES=50 %. We hypothesized, that hydrophobic substances like benzaldehyde and benzyl alcohol accumulate into the hydrophobic, upper DES-enriched phase. Technically, we repeated the experiments as mentioned above but this time, we added the components of interest to the system and took samples of each phase. Then, we extracted each phase with DCM and delivered the samples to the GC. From the results, >95 % of the hydrophobic components were extracted into the DES-enriched layer (Table 1). Although 25 mmol L−1 (based on one mL of total volume) of either product or substrate were used, the concentrations in the table are not suitable due to the unknown phase ratio (the exact volume of each phase) when separating the phases. However, the overall quantity clearly shows the distribution towards the DES-enriched layer.
Sample |
Concentration in AQ-enriched layer [mmol L−1], (quantity [%])[b] |
Concentration in DES-enriched layer [mmol L−1], (quantity [%])[b] |
|---|---|---|
Substrate, sample 1 |
0.8, (2) |
36.9, (98) |
Substrate, sample 2 |
0.9, (3) |
28.4, (97) |
Substrate, sample 3 |
0.8, (3) |
27.9, (97) |
Product, sample 1 |
1.7, (5) |
29.9, (95) |
Product, sample 2 |
1.5, (5) |
28.8, (95) |
Product, sample 3 |
1.7, (5) |
29.9, (95) |
- [a] TMS-DES composed of 50 mmol L−1 KPi (pH 6.0) and a mass fraction of wDES=50 % after separating the phases at 45 °C for five minutes and subsequent settling at 30 °C for 24 h. Samples 1, 2, 3 represent complete independent individual experiments. [b] Quantity values were calculated as: (concentration in the respective layer)/(sum of concentration in both layers).
To better understand the envisioned model reaction (benzaldehyde to benzyl alcohol, Figure 1), we investigated three different temperatures in the aqueous buffered system (Figure 6). As expected, the initial reaction rate increases with increasing temperature. Even if no detailed Michaelis–Menten kinetic studies are shown here, this statement can be supported by a closer look at the first hour of product-concentration of approximately 6.5 mmol L−1 at 20 °C, versus 7.5 mmol L−1 at 25 °C, and versus 9.8 mmol L−1 at 30 °C (Figure 6, time=1 h at each temperature). Nevertheless, we could prove with these findings that the reaction sufficiently proceeds at 20 °C where the respective DES-TMS shows monophasic conditions.
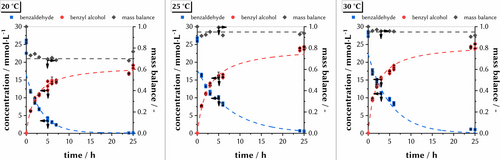
Progressive curves of buffer reactions at 20 °C (left), 25 °C (middle), and 30 °C (right). Reaction conditions: 25 mmol L−1 benzaldehyde, 50 mmol L−1 NADH, and 400 mg L−1 HLADH in 50 mmol L−1 KPi (pH 6.0). The progressive curves are a guide for the eyes without any mathematical relationship.
Up to this point, we have shown that the separation of biocatalyst and products is possible by simply changing the temperature of the DES-TMS under study. Subsequently, we performed a test reaction on a larger scale within the system. At the start of the reaction, 10 g of DES and 9 mL of 50 mmol L−1 KPi (pH 6.0) were used. Samples were taken from each phase at 30 °C, and to start the reaction, 1 mL HLADH solution was added and the system was cooled to 20 °C. For the DES-TMS, we anticipated similar reaction rates from the experiment where we determined the rates in the buffered system at 20 °C (Figure 6). A follow-up study will further investigate the actual enzyme kinetics in the monophasic DES-containing system. After 24 h, the system was heated to promote the phase change and we choose a phase separation time of 24 h to ensure a full separation. Again, samples were then taken from each phase. However, this equilibration time for phase separation was not optimized and we think it can be significantly shortened in further studies. The old DES phase was discarded, and the same amount of new DES was used together with new 10 mmol L−1 of benzaldehyde. In total, the enzyme was recycled three times. Within the method developing phase, we found that heating the system to 45 °C for five minutes significantly promoted the phase separation process. Afterwards, the system showed two stable layers at 30 °C (Figure 7).

A system of 50 mmol L−1 KPi (pH 6.0) and a mass fraction of wDES=50 % used within the recycling of the enzyme at 20 °C (left), at 30 °C with stirring (middle), and at 30 °C without stirring (right).
When calculating the concentrations used in the reactor, the calculation was always based on the total volume of 20 mL. When samples are taken from the two-phase system, the volume changes and hereby also the concentrations. If the phase separation occurs in a 1 : 1 ratio (10 mL for each phase), the concentrations would increase by the factor of 2. Since a simple extraction from the DES-phase failed, we diluted the whole sample in DCM and injected it to the GC. Due to this high loading of impurities, we decided to perform only one measurement per timepoint preventing the GC's column.
By measuring the volume of the recycled AQ-phase, a phase volume factor (mass of AQ-enriched phase/0.5⋅total mass) was used to process the GC data. Unfortunately, the yielded concentrations could not close the mass balance. However, we could show, that the recycling concept of the DES-TMS could be successfully established within this study (Table 2). After the substrate has homogenized in the system, we found 30 mmol L−1 of it in the DES-enriched phase and 1.1 mmol L−1 in the AQ-enriched phase (Table 2, entry 1). After the addition of the enzyme HLADH and 24 h of reaction time followed by the separation procedure of 5 min at 45 °C and subsequently 24 h at 30 °C, we observed a conversion of approximately 58 % (30 mmol L−1 to 12.7 mmol L−1 of substrate) yielding 20.6 mmol L−1 of benzyl alcohol (Table 2, entry 2). Even if the mass balance could not be closed during further recycling runs (Table 2, entries 3–5), we clearly observed a yield of benzyl alcohol. Further improvements will be performed taking a closer look at this challenge. It should be highlighted, that we observed only very low concentration in the AQ-enriched phase of the system of maximum 0.9 mmol L−1 of benzyl alcohol, and maximum 0.3 mmol L−1 of benzaldehyde. The decreased productivity of the enzyme may be either due to deactivation due to the long contact time within the system or/and that the NADH is consumed to a level that the reaction equilibrium is not favorable anymore. However, no measurement of NADH concentration was performed. Freshly prepared NADH could be added to the reaction system, or a cofactor regeneration system could be used in future studies. The use of catalytic amounts of the cofactor NADH is essential from an economic point of view to further optimize the system. Here, either a process with coupled substrates by adding ethanol or 2-propanol or a process with coupled enzymes by adding the enzyme glucose dehydrogenase and glucose is conceivable. Indeed, we observed higher yields if ethanol was used additionally as a cosubstrate in an aqueous system (99.6 % yield with 50 mmol L−1 of ethanol compared to 98.5 % at the same conditions without ethanol). However, careful investigations of the phase behavior by adding auxiliary components to the DES-TMS case are crucial (compare Figure 4), and we will answer these research questions in further studies. From this proof-of-concept experiment, we state that i) the reaction itself takes place, ii) the recycling of the enzyme is working, iii) the extraction of substrate/product into the DES-enriched phase, and of the enzyme into the AQ-enriched phase is successful (otherwise no second reaction would take place), and iv) the TMS behavior is very reproducible.
# |
Recycling runs |
Volume AQ [mL] |
Benzaldehyde, DES layer [mmol L−1] |
Benzyl alcohol, DES layer [mmol L−1] |
Benzaldehyde, AQ layer [mmol L−1] |
Benzyl alcohol, AQ layer [mmol L−1] |
|---|---|---|---|---|---|---|
1 |
Initial |
6.67 |
30.0 |
0.0 |
1.1 |
0.0 |
2 |
0 |
6.67 |
12.7 |
20.6 |
0.1 |
0.9 |
3 |
1 |
5.29 |
9.7 |
7.9 |
0.2 |
0.3 |
4 |
2 |
4.27 |
12.2 |
3.0 |
0.3 |
0.1 |
5 |
3 |
– |
10.6 |
1.4 |
0.3 |
0.1 |
- [a] Samples are measured on the GC and processed with the respective GC calibration method for either the AQ-enriched phase or the DES phase. Reaction time (temperature): 24 h (20 °C), phase separation time (temperature): 5 min (45 °C) followed by 24 h (30 °C), enzyme concentration: 400 mg L−1, NADH starting concentration: 50 mmol L−1.
Conclusion
Within our study, we successfully presented a recycling strategy of a liquid-immobilized horse liver alcohol dehydrogenase within a hydrophobic deep eutectic solvent-thermomorphic multiphasic system (DES-TMS). To the best of our knowledge, no studies on temperature-, buffer-, and pH-dependent binodal curves have been published at the time of this publication. We present here the very first example of extracting hydrophobic components and proteins into a DES-enriched and into an AQ-enriched layer, respectively, in one combined unit operation by simply changing the temperature. To the best of our knowledge, we presented the first example of a DES-TMS biocatalytic process intensification concept. If the DES is combined with ultrapure water, we observed a “U”-shaped binodal curve with a LCST-critical point of 25.5±0.5 °C at wDES=50 %. Nevertheless, the addition of potassium phosphate buffer salts significantly changes the shape of the binodal curves towards a “W”-shape. However, at wDES=50 %, the cloud points were determined to 25.5±0.5 °C (50 mmol L−1 KPi buffer, pH 7.5), 23.0±0.5 °C (50 mmol L−1 KPi buffer, pH 6.0), 23.5±0.5 °C (100 mmol L−1 KPi buffer, pH 7.5), and 22.5±0.5 °C (100 mmol L−1 KPi buffer, pH 6.0). Adding different additional components to a system of 50 mmol L−1 KPi (pH 6.0) and a mass fraction of wDES=50 % yielded in a decrease of the cloud point of approximately 0.026 °C per concentration unit for all compounds. The addition of BSA had no influence towards the binodal. Additionally, we estimate an extraction of BSA into the AQ-enriched layer of >95 % and the hydrophobic substrate (benzaldehyde) and educt (benzyl alcohol) accumulate >95 % in the DES-enriched layer. In a larger-scale experiment, the biocatalyst could be recycled by simply removing the DES phase for at least three recycling runs.
With our findings of a biocatalyzed reaction within the DES-TMS system as a combination of an extraction/reaction step in one unit operation, we believe to improve overall biocatalytic reactions in research as well in industrial applications. Low temperatures will protect sensitive biocatalysts and organic components, the extraction system will simplify the downstream processing and ensure a high recovery and reuse of the biocatalysts through liquid-immobilization. We specifically chose the enzyme HLADH because of its universal applicability and broad substrate specificity with excellent stereoselectivities. In future studies, we will investigate the reduction of prochiral ketones to obtain more valuable chiral secondary alcohols. In addition, mono- and bicyclic ketones can be reduced with good to very good specificity, which will also be part of further studies. In future follow-up studies, we will envisage similar hydrophobic DES systems with more conventional components, such as palmitic acid, camphor, citric acid, or sorbitol as hydrogen-bond donors and chlorine chloride, glycine, lactic acid, or dl-menthol as hydrogen-bond acceptors. If it were possible to remove the product from the DES, for example, by distillation, the DES could be reused in another cycle to reduce the cost of the whole process. Finally, many bio-based DES components are tailor made, renewable and biodegradable, originating from industrial waste, which reduces their overall economic and environmental impact.
Acknowledgements
S.K. and L.-E.M. thank the Independent Research Fund Denmark, PHOTOX-f project, grant No 9063-00031B, for the grant funding.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.