Total Synthesis of Starfish Cyclic Steroid Glycosides
Dr. Dapeng Zhu
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorMingyu Geng
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Biao Yu
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
School of Chemistry and Materials Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou, 310024 China
Search for more papers by this authorDr. Dapeng Zhu
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorMingyu Geng
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Biao Yu
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
School of Chemistry and Materials Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou, 310024 China
Search for more papers by this authorGraphical Abstract
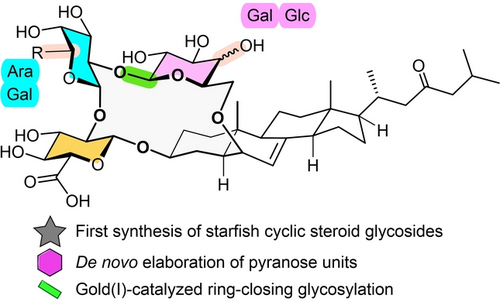
Enabled by de novo construction of the ether-linked hexopyranosyl units, the use of olefinic pyranoses as sugar precursors, and a gold(I)-catalyzed ring-closing glycosylation, the major starfish cyclic steroid glycosides, namely luzonicoside A and D and sepositoside A, have been synthesized for the first time.
Abstract
Starfishes have evolved with a special type of secondary metabolites, namely starfish saponins, to ward off various predators and parasites; among them, the starfish cyclic steroid glycosides stand out structurally, featuring a unique 16-membered ring formed by bridging the steroidal C3 and C6 with a trisaccharide. The rigid cyclic scaffold and the congested and vulnerable steroid-sugar etherate linkage present an unprecedented synthetic challenge. Here we report a collective total synthesis of the major starfish cyclic steroid glycosides, namely luzonicosides A (1) and D (2) and sepositoside A (3), with an innovative approach, which entails a de novo construction of the ether-linked hexopyranosyl units, use of olefinic pyranoses as sugar precursors, and a decisive ring-closing glycosylation under the mild gold(I)-catalyzed conditions.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202203239-sup-0001-misc_information.pdf7.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1V. A. Stonik, A. A. Kicha, T. V. Malyarenko, N. V. Ivanchina, Mar. Drugs 2020, 18, 584–613.
- 2J.-M. Xia, Z. Miao, C.-L. Xie, J.-W. Zhang, X.-W. Yang, Chem. Biodiversity 2020, 17, e1900638.
- 3F. De Simone, A. Dini, E. Finamore, L. Minale, C. Pizza, R. Riccio, F. Zollo, J. Chem. Soc. Perkin Trans. 1 1981, 1855–1862.
- 4R. Riccio, E. De Simone, A. Dini, L. Minale, C. Pizza, F. Senatore, F. Zollo, Tetrahedron Lett. 1981, 22, 1557–1560.
- 5R. Riccio, A. Dini, L. Minale, C. Pizza, F. Zollo, T. Sevenet, Experientia 1982, 38, 68–70.
- 6A. A. Kicha, A. I. Kalinovsky, T. V. Malyarenko, N. V. Ivanchina, P. S. Dmitrenok, E. S. Menchinskaya, E. A. Yurchenko, E. A. Pislyagin, D. L. Aminin, T. T. T. Huong, P. Q. Long, V. A. Stonik, J. Nat. Prod. 2015, 78, 1397–1405.
- 7T. V. Malyarenko, A. A. Kicha, N. V. Ivanchina, A. I. Kalinovsky, P. S. Dmitrenok, V. A. Stonik, Nat. Prod. Commun. 2016, 11, 1251–1252.
- 8J. Xie, N. Bogliotti, Chem. Rev. 2014, 114, 7678–7739.
- 9J. Rodriguez, S. O'Neill, M. A. Walczak, Nat. Prod. Rep. 2018, 35, 220–229.
- 10O. S. Malyarenko, S. A. Dyshlovoy, A. A. Kicha, N. V. Ivanchina, T. V. Malyarenko, B. Carsten, V. A. Gunhild, V. A. Stonik, S. P. Ermakova, Mar. Drugs 2017, 15, 227–238.
- 11G. Xiao, B. Yu, Chem. Eur. J. 2013, 19, 7708–7712.
- 12Y. Dai, B. Yu, Chem. Commun. 2015, 51, 13826–13829.
- 13D. Zhu, B. Yu, J. Am. Chem. Soc. 2015, 137, 15098–15101.
- 14X. Chen, X. Shao, W. Li, X. Zhang, B. Yu, Angew. Chem. Int. Ed. 2017, 56, 7648–7652; Angew. Chem. 2017, 129, 7756–7760.
- 15G. Xiao, X. Shao, D. Zhu, B. Yu, Nat. Prod. Rep. 2019, 36, 769–787.
- 16P. Xu, B. Yu, Adv. Carbohydr. Chem. Biochem. 2021, 79, 1–62.
- 17J. Chen, P. Koswatta, J. R. DeBergh, P. Fu, E. Pan, J. B. MacMillan, J. M. Ready, Chem. Sci. 2015, 6, 2932–2937.
- 18B.-F. Shi, PhD thesis, University of Chinese Academy of Sciences (Beijing, China), 2006.
- 19Y. Chen, PhD thesis, University of Chinese Academy of Sciences (Beijing, China), 2010.
- 20G. Xiao, PhD thesis, University of Chinese Academy of Sciences (Beijing, China), 2013.
- 21A. Z. Aljahdali, P. Shi, Y. Zhong, G. A. O'Doherty, Adv. Carbohydr. Chem. Biochem. 2013, 69, 55–123.
- 22R. S. Babu, G. A. O'Doherty, J. Am. Chem. Soc. 2003, 125, 12406–12407.
- 23W. Lim, J. Kim, Y. H. Rhee, J. Am. Chem. Soc. 2014, 136, 13618–13621.
- 24M. Kim, S. Kang, Y. H. Rhee, Angew. Chem. Int. Ed. 2016, 55, 9733–9737; Angew. Chem. 2016, 128, 9885–9889.
- 25
- 25aA. A. Scholte, M. H. An, M. L. Snapper, Org. Lett. 2006, 8, 4759–4762;
- 25bM. M. Ahmed, G. A. O'Doherty, Carbohydr. Res. 2006, 341, 1505–1521;
- 25cM. M. Ahmed, G. A. O'Doherty, J. Org. Chem. 2005, 70, 10576–10578;
- 25dM. M. Ahmed, G. A. O'Doherty, Tetrahedron Lett. 2005, 46, 4151–4155;
- 25eM. M. Ahmed, G. A. O'Doherty, Tetrahedron Lett. 2005, 46, 3015–3019;
- 25fM. M. Ahmed, B. P. Berry, T. J. Hunter, D. J. Tomcik, G. A. O'Doherty, Org. Lett. 2005, 7, 745–748.
- 26D. Ikuta, Y. Hirata, S. Wakamori, H. Shimada, Y. Tomabechi, Y. Kawasaki, K. Ikeuchi, T. Hagimori, S. Matsumoto, H. Yamada, Science 2019, 364, 674–677.
- 27X. G. Jia, A. V. Demchenko, Beilstein J. Org. Chem. 2017, 13, 2028–2048.
- 28S.-H. Son, N. Yanagiya, J.-i. Furukawa, N. Sakairi, Synlett 2009, 2957–2960.
- 29M. Nawój, A. Grobelny, J. Mlynarski, Eur. J. Org. Chem. 2020, 47–51.
- 30M. Wakao, K. Fukase, S. Kusumoto, J. Org. Chem. 2002, 67, 8182–8190.
- 31S. J. Danishefsky, M. T. Bilodeau, Angew. Chem. Int. Ed. Engl. 1996, 35, 1380–1419; Angew. Chem. 1996, 108, 1482–1522.
- 32Y. Tang, J. Li, Y. Zhu, Y. Li, B. Yu, J. Am. Chem. Soc. 2013, 135, 18396–18405.
- 33B. Yu, Acc. Chem. Res. 2018, 51, 507–516.
- 34G. C. Vougioukalakis, R. H. Grubbs, Chem. Rev. 2010, 110, 1746–1787.
- 35A. K. Chatterjee, T. L. Choi, D. P. Sanders, R. H. Grubbs, J. Am. Chem. Soc. 2003, 125, 11360–11370.
- 36T. R. Hoye, H. Zhao, Org. Lett. 1999, 1, 1123–1125.
- 37Y. A. Lin, B. G. Davis, Beilstein J. Org. Chem. 2010, 6, 1219–1228.
- 38H. C. Kolb, M. S. VanNieuwenhze, K. B. Sharpless, Chem. Rev. 1994, 94, 2483–2547.
- 39K. B. Sharpless, W. Amberg, Y. L. Bennani, G. A. Crispino, J. Hartung, K.-S. Jeong, H.-L. Kwong, K. Morikawa, Z.-M. Wang, D. Xu, X.-L. Zhang, J. Org. Chem. 1992, 57, 2768–2771.
- 40E. J. Corey, R. A. Ruden, J. Org. Chem. 1973, 38, 834–835.
- 41H. Dong, Z. Pei, O. Ramström, J. Org. Chem. 2006, 71, 3306–3309.
- 42H. Hattori, J. Roesslein, P. Caspers, K. Zerbe, H. Miyatake-Ondozabal, D. Ritz, G. Rueedi, K. Gademann, Angew. Chem. Int. Ed. 2018, 57, 11020–11024; Angew. Chem. 2018, 130, 11186–11190.
- 43K. Kamaike, H. Takahashi, T. Kakinuma, K. Morohoshi, Y. Ishido, Tetrahedron Lett. 1997, 38, 6857–6860.
- 44H. Jona, H. Mandai, W. Chavasiri, K. Takeuchi, T. Mukaiyama, Bull. Chem. Soc. Jpn. 2002, 75, 291–309.
- 45N. Nakajima, K. Horita, R. Abe, O. Yonemitsu, Tetrahedron Lett. 1988, 29, 4139–4142.
- 46H. M. Christensen, S. Oscarson, H. H. Jensen, Carbohydr. Res. 2015, 408, 51–95.
- 47S. van der Vorm, T. Hansen, J. M. A. van Hengst, H. S. Overkleeft, G. A. van der Marel, J. D. C. Codée, Chem. Soc. Rev. 2019, 48, 4688–4706.
- 48E. J. Corey, P. B. Hopkins, Tetrahedron Lett. 1982, 23, 1979–1982.
- 49R. R. Schmidt, J. Michel, Angew. Chem. Int. Ed. Engl. 1980, 19, 731–732; Angew. Chem. 1980, 92, 763–764.
- 50
- 50aG. A. Crispino, K.-S. Jeong, H. C. Kolb, Z.-M. Wang, D. Xu, K. B. Sharpless, J. Org. Chem. 1993, 58, 3785–3786;
- 50bH.-Y. L. Wang, G. A. O'Doherty, Chem. Commun. 2011, 47, 10251–10253;
- 50cJ. W. Hinds, S. B. McKenna, E. U. Sharif, H.-Y. L. Wang, N. G. Akhmedov, G. A. O'Doherty, ChemMedChem 2013, 8, 63–69.