Visible-Light-Induced, Single-Metal-Catalyzed, Directed C−H Functionalization: Metal-Substrate-Bound Complexes as Light-Harvesting Agents
Chao Pei
RWTH Aachen University, Institute of Organic Chemistry, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorClaire Empel
RWTH Aachen University, Institute of Organic Chemistry, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Rene M. Koenigs
RWTH Aachen University, Institute of Organic Chemistry, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorChao Pei
RWTH Aachen University, Institute of Organic Chemistry, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorClaire Empel
RWTH Aachen University, Institute of Organic Chemistry, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Rene M. Koenigs
RWTH Aachen University, Institute of Organic Chemistry, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorGraphical Abstract
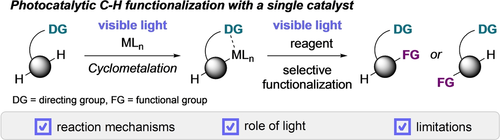
Recent advances in photochemical C−H functionalization reactions using a single metal catalyst to enable sustainable strategies for the functionalization of C−H bonds under ambient conditions without an external photosensitizer are highlighted in this Minireview. Reaction mechanisms and the role of visible light are described, and future perspectives and challenges to stimulate future research directions in the field of light-mediated C−H functionalization are discussed.
Abstract
C−H functionalization represents one of the most rapidly advancing areas in organic synthesis and is regarded as one of the key concepts to minimize the ecological and economic footprint of organic synthesis. The ubiquity and low reactivity of C−H bonds in organic molecules, however, poses several challenges, and often necessitates harsh reaction conditions to achieve this goal, although it is highly desirable to achieve C−H functionalization reactions under mild conditions. Recently, several reports uncovered a conceptually new approach towards C−H functionalization, where a single transition-metal complex can be used as both the photosensitizer and catalyst to promote C−H bond functionalization in the absence of an exogeneous photosensitizer. In this Minireview, we will provide an overview on recent achievements in C−H functionalization reactions, with an emphasis on the photochemical modulation of the reaction mechanism using such catalysts.
Conflict of interest
The authors declare no conflict of interest.
References
- 1T. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147–1169.
- 2M. P. Doyle, R. Duffy, M. Ratnikov, L. Zhou, Chem. Rev. 2010, 110, 704–724.
- 3H. M. L. Davies, D. Morton, Chem. Soc. Rev. 2011, 40, 1857.
- 4K. R. Campos, Chem. Soc. Rev. 2007, 36, 1069–1084.
- 5C. Empel, R. M. Koenigs, Synlett 2019, 30, 1929–1934.
- 6C.-S. Wang, P. H. Dixneuf, J.-F. Soulé, Chem. Rev. 2018, 118, 7532–7585.
- 7I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy, J. F. Hartwig, Chem. Rev. 2010, 110, 890–931.
- 8R. Shang, L. Ilies, E. Nakamura, Chem. Rev. 2017, 117, 9086–9139.
- 9T. Gensch, M. J. James, T. Dalton, F. Glorius, Angew. Chem. Int. Ed. 2018, 57, 2296–2306; Angew. Chem. 2018, 130, 2318–2328.
- 10Y. Park, Y. Kim, S. Chang, Chem. Rev. 2017, 117, 9247–9301.
- 11T. Gensch, M. N. Hopkinson, F. Glorius, J. Wencel-Delord, Chem. Soc. Rev. 2016, 45, 2900–2936.
- 12L. Ackermann, Chem. Rev. 2011, 111, 1315–1345.
- 13J. B. Williamson, S. E. Lewis, R. R. Johnson, I. M. Manning, F. A. Leibfarth, Angew. Chem. Int. Ed. 2019, 58, 8654–8668; Angew. Chem. 2019, 131, 8746–8761.
- 14Y. Segawa, T. Maekawa, K. Itami, Angew. Chem. Int. Ed. 2015, 54, 66–81; Angew. Chem. 2015, 127, 68–83.
- 15J. Wencel-Delord, F. Glorius, Nat. Chem. 2013, 5, 369–375.
- 16R. R. Karimov, J. F. Hartwig, Angew. Chem. Int. Ed. 2018, 57, 4234–4241; Angew. Chem. 2018, 130, 4309–4317.
- 17W. R. Gutekunst, P. S. Baran, Chem. Soc. Rev. 2011, 40, 1976.
- 18T. Cernak, K. D. Dykstra, S. Tyagarajan, P. Vachal, S. W. Krska, Chem. Soc. Rev. 2016, 45, 546–576.
- 19D. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624–655.
- 20G. Rouquet, N. Chatani, Angew. Chem. Int. Ed. 2013, 52, 11726–11743; Angew. Chem. 2013, 125, 11942–11959.
- 21F. Zhang, D. R. Spring, Chem. Soc. Rev. 2014, 43, 6906–6919.
- 22L. Guillemard, J. Wencel-Delord, Beilstein J. Org. Chem. 2020, 16, 1754–1804.
- 23Y. Tang, Y. Li, F. Tao, Chem. Soc. Rev. 2022, 51, 376–423.
- 24Z. Yang, M. L. Stivanin, I. D. Jurberg, R. M. Koenigs, Chem. Soc. Rev. 2020, 49, 6833–6847.
- 25C. Empel, S. Jana, R. M. Koenigs, Molecules 2020, 25, 880.
- 26D. Zhu, L. Chen, H. Fan, Q. Yao, S. Zhu, Chem. Soc. Rev. 2020, 49, 908–950.
- 27H. M. L. Davies, J. R. Manning, Nature 2008, 451, 417–424.
- 28F. Collet, C. Lescot, P. Dauban, Chem. Soc. Rev. 2011, 40, 1926.
- 29G. Dequirez, V. Pons, P. Dauban, Angew. Chem. Int. Ed. 2012, 51, 7384–7395; Angew. Chem. 2012, 124, 7498–7510.
- 30O. Baslé, Curr. Opin. Green Sustainable Chem. 2021, 32, 100539.
- 31R. Kancherla, K. Muralirajan, A. Sagadevan, M. Rueping, Trends Chem. 2019, 1, 510–523.
- 32P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz, L. Ackermann, Chem. Rev. 2019, 119, 2192–2452.
- 33A. Wiebe, T. Gieshoff, S. Möhle, E. Rodrigo, M. Zirbes, S. R. Waldvogel, Angew. Chem. Int. Ed. 2018, 57, 5594–5619; Angew. Chem. 2018, 130, 5694–5721.
- 34M. Yan, Y. Kawamata, P. S. Baran, Chem. Rev. 2017, 117, 13230–13319.
- 35N. Sauermann, T. H. Meyer, Y. Qiu, L. Ackermann, ACS Catal. 2018, 8, 7086–7103.
- 36S. Tang, Y. Liu, A. Lei, Chem 2018, 4, 27–45.
- 37J. Yoshida, A. Shimizu, R. Hayashi, Chem. Rev. 2018, 118, 4702–4730.
- 38M. Parasram, V. Gevorgyan, Chem. Soc. Rev. 2017, 46, 6227–6240.
- 39K. P. S. Cheung, S. Sarkar, V. Gevorgyan, Chem. Rev. 2022, 122, 1543–1625.
- 40P. Chuentragool, D. Kurandina, V. Gevorgyan, Angew. Chem. Int. Ed. 2019, 58, 11586–11598; Angew. Chem. 2019, 131, 11710–11722.
- 41J. Thongpaen, R. Manguin, V. Dorcet, T. Vives, C. Duhayon, M. Mauduit, O. Baslé, Angew. Chem. Int. Ed. 2019, 58, 15244–15248; Angew. Chem. 2019, 131, 15388–15392.
- 42L. Yang, K. Semba, Y. Nakao, Angew. Chem. Int. Ed. 2017, 56, 4853–4857; Angew. Chem. 2017, 129, 4931–4935.
- 43J. Tanaka, Y. Nagashima, A. J. Araujo Dias, K. Tanaka, J. Am. Chem. Soc. 2021, 143, 11325–11331.
- 44P. Gandeepan, J. Koeller, K. Korvorapun, J. Mohr, L. Ackermann, Angew. Chem. Int. Ed. 2019, 58, 9820–9825; Angew. Chem. 2019, 131, 9925–9930.
- 45A. Sagadevan, M. F. Greaney, Angew. Chem. Int. Ed. 2019, 58, 9826–9830; Angew. Chem. 2019, 131, 9931–9935.
- 46N. Hofmann, L. Ackermann, J. Am. Chem. Soc. 2013, 135, 5877–5884.
- 47L. Ackermann, P. Novák, Org. Lett. 2009, 11, 4966–4969.
- 48J. Struwe, K. Korvorapun, A. Zangarelli, L. Ackermann, Chem. Eur. J. 2021, 27, 16237–16241.
- 49A. Sagadevan, A. Charitou, F. Wang, M. Ivanova, M. Vuagnat, M. F. Greaney, Chem. Sci. 2020, 11, 4439–4443.
- 50K. Korvorapun, J. Struwe, R. Kuniyil, A. Zangarelli, A. Casnati, M. Waeterschoot, L. Ackermann, Angew. Chem. Int. Ed. 2020, 59, 18103–18109; Angew. Chem. 2020, 132, 18259–18265.
- 51R. Boyaala, R. Touzani, T. Roisnel, V. Dorcet, E. Caytan, D. Jacquemin, J. Boixel, V. Guerchais, H. Doucet, J.-F. Soulé, ACS Catal. 2019, 9, 1320–1328.
- 52J. Hubrich, T. Himmler, L. Rodefeld, L. Ackermann, ACS Catal. 2015, 5, 4089–4093.
- 53W. Li, P. B. Arockiam, C. Fischmeister, C. Bruneau, P. H. Dixneuf, Green Chem. 2011, 13, 2315.
- 54S. Jana, C. Pei, S. B. Bahukhandi, R. M. Koenigs, Chem Catal. 2021, 13, 467–479.
- 55G. F. Manbeck, E. Fujita, K. J. Brewer, J. Am. Chem. Soc. 2017, 139, 7843–7854.
- 56J. Kim, D. Kim, S. Chang, J. Am. Chem. Soc. 2020, 142, 19052–19057.
- 57K. Shin, Y. Park, M.-H. Baik, S. Chang, Nat. Chem. 2018, 10, 218–224.