Concurrent Prebiotic Formation of Nucleoside-Amidophosphates and Nucleoside-Triphosphates Potentiates Transition from Abiotic to Biotic Polymerization
Dr. Huacan Lin
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
NSF-NASA Center for Chemical Evolution, Atlanta, GA, 30332 USA
Search for more papers by this authorDr. Eddy I. Jiménez
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorJoshua T. Arriola
Department of Chemistry and Biochemistry, UC San Diego, 9500 Gilman Drive, La Jolla, CA, 92037 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Ulrich F. Müller
Department of Chemistry and Biochemistry, UC San Diego, 9500 Gilman Drive, La Jolla, CA, 92037 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Ramanarayanan Krishnamurthy
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
NSF-NASA Center for Chemical Evolution, Atlanta, GA, 30332 USA
Search for more papers by this authorDr. Huacan Lin
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
NSF-NASA Center for Chemical Evolution, Atlanta, GA, 30332 USA
Search for more papers by this authorDr. Eddy I. Jiménez
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorJoshua T. Arriola
Department of Chemistry and Biochemistry, UC San Diego, 9500 Gilman Drive, La Jolla, CA, 92037 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Ulrich F. Müller
Department of Chemistry and Biochemistry, UC San Diego, 9500 Gilman Drive, La Jolla, CA, 92037 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Ramanarayanan Krishnamurthy
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
NSF-NASA Center for Chemical Evolution, Atlanta, GA, 30332 USA
Search for more papers by this authorIn memory of Leslie Orgel
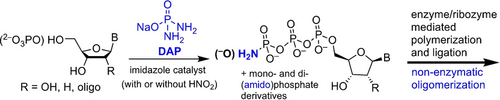
Graphical Abstract
The DAP mediated prebiotic phosphorylation of nucleosides/nucleotides/oligonucleotides produces a spectrum of the corresponding mono-, di-, and tri(amido)phosphorylated derivatives which are substrates for both prebiotic-oligomerization and biotic-polymerization/ligation. The compatibility of the phosphorylation and activation chemistry across a broad spectrum of (oligo)nucleos(t)ide structures suggests a smoother transition from chemistry to biology.
Abstract
Polymerization of nucleic acids in biology utilizes 5′-nucleoside triphosphates (NTPs) as substrates. The prebiotic availability of NTPs has been unresolved and other derivatives of nucleoside-monophosphates (NMPs) have been studied. However, this latter approach necessitates a change in chemistries when transitioning to biology. Herein we show that diamidophosphate (DAP), in a one-pot amidophosphorylation-hydrolysis setting converts NMPs into the corresponding NTPs via 5′-nucleoside amidophosphates (NaPs). The resulting crude mixture of NTPs are accepted by proteinaceous- and ribozyme-polymerases as substrates for nucleic acid polymerization. This phosphorylation also operates at the level of oligonucleotides enabling ribozyme-mediated ligation. This one-pot protocol for simultaneous generation of NaPs and NTPs suggests that the transition from prebiotic-phosphorylation and oligomerization to an enzymatic processive-polymerization can be more continuous than previously anticipated.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202113625-sup-0001-misc_information.pdf23.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. P. Robertson, G. F. Joyce, Cold Spring Harbor Perspect. Biol. 2012, 4, a003608;
- 1bT. R. Cech, Cold Spring Harbor Perspect. Biol. 2012, 4, a006742;
- 1cA. Lazcano, BIO Web of Conferences 2015, 4, 00013;
10.1051/bioconf/20150400013 Google Scholar
- 1dJ. P. Dworkin, A. Lazcano, L. Miller Stanley, J. Theor. Biol. 2003, 222, 127–134;
- 1eJ. Xu, V. Chmela, N. J. Green, D. A. Russell, M. J. Janicki, R. W. Gora, R. Szabla, A. D. Bond, J. D. Sutherland, Nature 2020, 582, 60–66.
- 2M. Yadav, R. Kumar, R. Krishnamurthy, Chem. Rev. 2020, 120, 4766–4805.
- 3
- 3aL. E. Orgel, Origins Life Evol. Biospheres 2003, 33, 211–218;
- 3bA. Pressman, C. Blanco, I. A. Chen, Curr. Biol. 2015, 25, R953–R963.
- 4
- 4aE. I. Jiménez, C. Gibard, R. Krishnamurthy, Angew. Chem. Int. Ed. 2021, 60, 10775–10783; Angew. Chem. 2021, 133, 10870–10878;
- 4bJ. Xu, N. J. Green, C. Gibard, R. Krishnamurthy, J. D. Sutherland, Nat. Chem. 2019, 11, 457–462.
- 5
- 5aR. Lohrmann, J. Mol. Evol. 1975, 6, 237–252;
- 5bG. J. Handschuh, R. Lohrmann, L. E. Orgel, J. Mol. Evol. 1973, 2, 251–262;
- 5cR. Österberg, L. E. Orgel, R. Lohrmann, J. Mol. Evol. 1973, 2, 231–234;
- 5dR. Reimann, G. Zubay, Origins Life Evol. Biospheres 1999, 29, 229–247;
- 5eH. J. Kim, S. A. Benner, Astrobiology 2021, 21, 298–306;
- 5fF. Chizzolini, A. D. Kent, L. F. M. Passalacqua, A. Luptak, ChemBioChem 2021, 22, 2098–2101.
- 6R. Rohatgi, D. P. Bartel, J. W. Szostak, J. Am. Chem. Soc. 1996, 118, 3340–3344.
- 7
- 7aJ. Oró, E. Stephen-Sherwood in Cosmochemical Evolution and the Origins of Life, Vol. 1 (Eds.: J. Oró, S. L. Miller, C. Ponnamperuma, R. S. Young), Springer, Dordrecht, 1974, pp. 159–172;
- 7bL. E. Orgel, Nature 1992, 358, 203–209;
- 7cL. E. Orgel, Crit. Rev. Biochem. Mol. Biol. 2004, 39, 99–123;
- 7dT. Walton, W. Zhang, L. Li, C. P. Tam, J. W. Szostak, Angew. Chem. Int. Ed. 2019, 58, 10812–10819; Angew. Chem. 2019, 131, 10926–10933;
- 7eE. Kervio, M. Sosson, C. Richert, Nucleic Acids Res. 2016, 44, 5504–5514;
- 7fP. G. Higgs, Life 2016, 6, 24.
- 8
- 8aD. Bartel, J. Szostak, Science 1993, 261, 1411–1418;
- 8bG. F. Dolan, A. Akoopie, U. F. Muller, PLoS One 2015, 10, e0142559.
- 9
- 9aR. Krishnamurthy, S. Guntha, A. Eschenmoser, Angew. Chem. Int. Ed. 2000, 39, 2281–2285;
10.1002/1521-3773(20000703)39:13<2281::AID-ANIE2281>3.0.CO;2-2 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 2369–2373;
- 9bM. Karki, C. Gibard, S. Bhowmik, R. Krishnamurthy, Life 2017, 7, 32;
- 9cA. Osumah, R. Krishnamurthy, ChemBioChem 2021, 22, 3001–3009.
- 10
- 10aC. Gibard, S. Bhowmik, M. Karki, E. K. Kim, R. Krishnamurthy, Nat. Chem. 2018, 10, 212–217;
- 10bC. Gibard, I. B. Gorrell, E. I. Jimenez, T. P. Kee, M. A. Pasek, R. Krishnamurthy, Angew. Chem. Int. Ed. 2019, 58, 8151–8155; Angew. Chem. 2019, 131, 8235–8239.
- 11E. Y. Song, E. I. Jimenez, H. Lin, K. Le Vay, R. Krishnamurthy, H. Mutschler, Angew. Chem. Int. Ed. 2021, 60, 2952–2957; Angew. Chem. 2021, 133, 2988–2993.
- 12L. Li, N. Prywes, C. P. Tam, D. K. O'Flaherty, V. S. Lelyveld, E. C. Izgu, A. Pal, J. W. Szostak, J. Am. Chem. Soc. 2017, 139, 1810–1813.
- 13J. Tomasz, J. Carbohydr. Nucleosides Nucleotides 1981, 8, 557–572.
- 14A. Simoncsits, J. Tomasz, Nucleic Acids Res. 1975, 2, 1223–1233.
- 15J. Kua, J. Bada, Origins Life Evol. Biospheres 2011, 41, 553—558.
- 16
- 16aW. P. Jencks, M. Gilchrist, J. Am. Chem. Soc. 1964, 86, 1410–1417;
- 16bZ. Jerman, H. Rathánová, F. Markalous, Collect. Czech. Chem. Commun. 1969, 34, 3991–3994.
- 17
- 17aD. P. Summers, S. Chang, Nature 1993, 365, 630–633;
- 17bD. P. Summers, Origins Life Evol. Biospheres 1999, 29, 33–46;
- 17cS. Ranjan, Z. R. Todd, P. B. Rimmer, D. D. Sasselov, A. R. Babbin, Geochem. Geophys. Geosyst. 2019, 20, 2021–2039.
- 18S. Becker, J. Feldmann, S. Wiedemann, H. Okamura, C. Schneider, K. Iwan, A. Crisp, M. Rossa, T. Amatov, T. Carell, Science 2019, 366, 76–82.
- 19Y. Yamagata, T. Matsukawa, T. Mohri, K. Inomata, Nature 1979, 282, 284–286.
- 20M. Maslak, C. T. Martin, Biochemistry 1994, 33, 6918–6924.
- 21W. K. Johnston, P. J. Unrau, M. S. Lawrence, M. E. Glasner, D. P. Bartel, Science 2001, 292, 1319–1325.
- 22
- 22aR. Lohrmann, L. E. Orgel, Science 1971, 171, 490–494;
- 22bR. Lohrmann, L. E. Orgel, Science 1968, 161, 64–66.
- 23J. Attwater, S. Tagami, M. Kimoto, K. Butler, E. T. Kool, J. Wengel, P. Herdewijn, I. Hirao, P. Holliger, Chem. Sci. 2013, 4, 2804–2814.
- 24E. Janzen, C. Blanco, H. Peng, J. Kenchel, I. A. Chen, Chem. Rev. 2020, 120, 4879–4897.
- 25J. E. Moretti, U. F. Müller, Nucleic Acids Res. 2014, 42, 4767–4778.
- 26J. Rogers, G. F. Joyce, RNA 2001, 7, 395–404.
- 27
- 27aA. W. Schwartz, J. Chem. Soc. D 1969, 1393a;
10.1039/C2969001393A Google Scholar
- 27bC. Cheng, C. Fan, R. Wan, C. Tong, Z. Miao, J. Chen, Y. Zhao, Origins Life Evol. Biospheres 2002, 32, 219–224.
- 28K. Gao, L. E. Orgel, Origins Life Evol. Biospheres 2000, 30, 45–51.
- 29R. Krishnamurthy, Chem. Eur. J. 2018, 24, 16708–16715.
- 30P. A. Monnard, D. W. Deamer, Origins Life Evol. Biospheres 2001, 31, 147–155.
- 31
- 31aS. Bhowmik, R. Krishnamurthy, Nat. Chem. 2019, 11, 1009–1018;
- 31bJ. Xu, N. J. Green, D. A. Russell, Z. Liu, J. D. Sutherland, J. Am. Chem. Soc. 2021, 143, 14482–14486.
- 32
- 32aI. Zlatev, T. Lavergne, F. Debart, J. J. Vasseur, M. Manoharan, F. Morvan, Org. Lett. 2010, 12, 2190–2193;
- 32bJ. Singh, A. Ripp, T. M. Haas, D. Qiu, M. Keller, P. A. Wender, J. S. Siegel, K. K. Baldridge, H. J. Jessen, J. Am. Chem. Soc. 2019, 141, 15013–15017.
- 33M. W. Powner, S. L. Zheng, J. W. Szostak, J. Am. Chem. Soc. 2012, 134, 13889–13895.