Tetraaryldiborane(4) Can Emit Dual Fluorescence Responding to the Structural Change around the B–B Bond
Corresponding Author
Prof. Dr. Yoshiaki Shoji
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Search for more papers by this authorDr. Naoki Tanaka
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Present address: Department of Applied Chemistry, Graduate School of Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395 Japan
International Institute for Carbon-Neutral Energy Research, (WPI-I2CNER), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395 Japan
Search for more papers by this authorCorresponding Author
Dr. Yasuhiro Ikabata
Waseda Research Institute for Science and Engineering, Waseda University, Tokyo, 169-8555 Japan
Present address: Information and Media Center, Toyohashi University of Technology, 1-1 Hibarigaoka, Tempaku-cho, Toyohashi, Aichi, 441-8580 Japan
Search for more papers by this authorDr. Hayato Sakai
Department of Chemistry, Faculty of Science and Technology, Keio University, Yokohama, 223-8522 Japan
Search for more papers by this authorProf. Dr. Taku Hasobe
Department of Chemistry, Faculty of Science and Technology, Keio University, Yokohama, 223-8522 Japan
Search for more papers by this authorProf. Dr. Norbert Koch
Institut für Physik and IRIS Adlershof, Humboldt-Universität zu Berlin, Berlin, 12489 Germany
Search for more papers by this authorProf. Dr. Hiromi Nakai
Waseda Research Institute for Science and Engineering, Waseda University, Tokyo, 169-8555 Japan
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, Tokyo, 169-8555 Japan
Search for more papers by this authorProf. Dr. Takanori Fukushima
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Yoshiaki Shoji
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Search for more papers by this authorDr. Naoki Tanaka
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Present address: Department of Applied Chemistry, Graduate School of Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395 Japan
International Institute for Carbon-Neutral Energy Research, (WPI-I2CNER), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395 Japan
Search for more papers by this authorCorresponding Author
Dr. Yasuhiro Ikabata
Waseda Research Institute for Science and Engineering, Waseda University, Tokyo, 169-8555 Japan
Present address: Information and Media Center, Toyohashi University of Technology, 1-1 Hibarigaoka, Tempaku-cho, Toyohashi, Aichi, 441-8580 Japan
Search for more papers by this authorDr. Hayato Sakai
Department of Chemistry, Faculty of Science and Technology, Keio University, Yokohama, 223-8522 Japan
Search for more papers by this authorProf. Dr. Taku Hasobe
Department of Chemistry, Faculty of Science and Technology, Keio University, Yokohama, 223-8522 Japan
Search for more papers by this authorProf. Dr. Norbert Koch
Institut für Physik and IRIS Adlershof, Humboldt-Universität zu Berlin, Berlin, 12489 Germany
Search for more papers by this authorProf. Dr. Hiromi Nakai
Waseda Research Institute for Science and Engineering, Waseda University, Tokyo, 169-8555 Japan
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, Tokyo, 169-8555 Japan
Search for more papers by this authorProf. Dr. Takanori Fukushima
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 Japan
Search for more papers by this authorGraphical Abstract
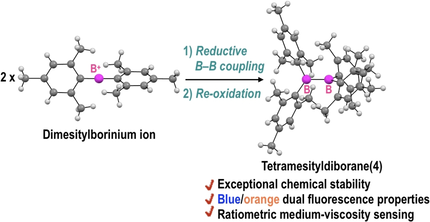
Newly synthesized tetramesityldiborane(4) shows exceptional chemical stability and blue/orange dual fluorescence capability associated with the B−B bond rotation in the excited state. The relative intensity of the dual fluorescence is sensitive to the viscosity of the medium, highlighting the potential of this diborane(4) as a ratiometric viscosity sensor.
Abstract
We report the successful synthesis of tetramesityldiborane(4) (Mes4B2) through the reductive coupling of a dimesitylborinium ion. Owing to the steric protection conferred by the mesityl groups, Mes4B2 shows exceptional chemical stability and remains intact in water. Single-crystal X-ray analysis revealed that Mes4B2 has an orthogonal geometry, where the B–B center is completely hidden by the mesityl groups. Remarkably, Mes4B2 emits dual fluorescence at 460 and 620 nm, both in solution and in the solid state. Theoretical calculations showed that Mes4B2 in the excited S1 state adopts a twisted or planar geometry, which is responsible for the shorter- or longer-wavelength fluorescence, respectively. The intensity ratio of the dual fluorescence is sensitive to the viscosity of the medium, which suggests that Mes4B2 has potential as a ratiometric viscosity sensor.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202113549-sup-0001-cif.zip590.6 KB | Supporting Information |
| anie202113549-sup-0001-misc_information.pdf1.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aE. C. Neeve, S. J. Geier, I. A. I. Mkhalid, S. A. Westcott, T. B. Marder, Chem. Rev. 2016, 116, 9091–9161;
- 1bM. Arrowsmith, H. Braunschweig, T. E. Stennett, Angew. Chem. Int. Ed. 2017, 56, 96–115; Angew. Chem. 2017, 129, 100–120;
- 1cH. Budy, J. Gilmer, T. Trageser, M. Wagner, Eur. J. Inorg. Chem. 2020, 4148–4162;
- 1dL. Kong, C. Cui, Synlett 2021, 32, 1316–1322.
- 2
- 2aY. Shoji, T. Matsuo, D. Hashizume, H. Fueno, K. Tanaka, K. Tamao, J. Am. Chem. Soc. 2010, 132, 8258–8260;
- 2bY. Shoji, T. Matsuo, D. Hashizume, M. J. Gutmann, H. Fueno, K. Tanaka, K. Tamao, J. Am. Chem. Soc. 2011, 133, 11058–11061;
- 2cY. Shoji, S. Kaneda, H. Fueno, K. Tanaka, K. Tamao, D. Hashizume, T. Matsuo, Chem. Lett. 2014, 43, 1587–1589.
- 3
- 3aT. Thiess, G. Bélanger-Chabot, F. Fantuzzi, M. Michel, M. Ernst, B. Engels, H. Braunschweig, Angew. Chem. Int. Ed. 2020, 59, 15480–15486; Angew. Chem. 2020, 132, 15608–15614;
- 3bC. Brunecker, J. H. Müssig, M. Arrowsmith, F. Fantuzzi, A. Stoy, J. Böhnke, A. Hofmann, R. Bertermann, B. Engels, H. Braunschweig, Chem. Eur. J. 2020, 26, 8518–8523;
- 3cJ. Seufert, E. Welz, I. Krummenacher, V. Paprocki, J. Böhnke, S. Hagspiel, R. D. Dewhurst, R. Tacke, B. Engels, H. Braunschweig, Angew. Chem. Int. Ed. 2018, 57, 10752–10755; Angew. Chem. 2018, 130, 10912–10915;
- 3dH. Braunschweig, A. Damme, T. Kupfer, Eur. J. Inorg. Chem. 2010, 4423–4426.
- 4
- 4aA. Moezzi, M. M. Olmstead, P. P. Power, J. Am. Chem. Soc. 1992, 114, 2715–2717;
- 4bA. Moezzi, M. M. Olmstead, R. A. Bartlett, P. P. Power, Organometallics 1992, 11, 2383–2388.
- 5
- 5aN. Tsukahara, H. Asakawa, K.-H. Lee, Z. Lin, M. Yamashita, J. Am. Chem. Soc. 2017, 139, 2593–2596;
- 5bY. Katsuma, N. Tsukahara, L. Wu, Z. Lin, M. Yamashita, Angew. Chem. Int. Ed. 2018, 57, 6109–6114; Angew. Chem. 2018, 130, 6217–6222;
- 5cY. Katsuma, L. Wu, Z. Lin, S. Akiyama, M. Yamashita, Angew. Chem. Int. Ed. 2019, 58, 317–321; Angew. Chem. 2019, 131, 323–327;
- 5dS. Akiyama, K. Yamada, M. Yamashita, Angew. Chem. Int. Ed. 2019, 58, 11806–11810; Angew. Chem. 2019, 131, 11932–11936;
- 5eS. Akiyama, S. Ikemoto, S. Muratsugu, M. Tada, M. Yamashita, Organometallics 2020, 39, 500–504;
- 5fA. Suzuki, X. Guo, Z. Lin, M. Yamashita, Chem. Sci. 2021, 12, 917–928.
- 6T. Araki, M. Hirai, A. Wakamiya, W. E. Piers, S. Yamaguchi, Chem. Lett. 2017, 46, 1714.
- 7F. Ge, X. Tao, C. G. Daniliuc, G. Kehr, G. Erker, Angew. Chem. Int. Ed. 2018, 57, 14570–14574; Angew. Chem. 2018, 130, 14778–14782.
- 8
- 8aA. Hübner, M. A. Bolte, H.-W. Lerner, M. Wagner, Angew. Chem. Int. Ed. 2014, 53, 10408–10411; Angew. Chem. 2014, 126, 10576–10579;
- 8bA. Hübner, T. Kaese, M. Diefenbach, B. Endeward, M. Bolte, H.-W. Lerner, M. C. Holthausen, M. Wagner, J. Am. Chem. Soc. 2015, 137, 3705–3714;
- 8cT. Kaese, A. Hübner, M. Bolte, H.-W. Lerner, M. Wagner, J. Am. Chem. Soc. 2016, 138, 6224–6233;
- 8dH. Budy, T. Kaese, M. Bolte, H.-W. Lerner, M. Wagner, Angew. Chem. Int. Ed. 2021, 60, 19397–19405; Angew. Chem. 2021, 133, 19546–19554.
- 9
- 9aL. L. Cao, D. W. Stephan, Organometallics 2017, 36, 3163–3170;
- 9bK. L. Bamford, Z.-W. Qu, D. W. Stephan, Angew. Chem. Int. Ed. 2021, 60, 8532–8536; Angew. Chem. 2021, 133, 8613–8617.
- 10
- 10aY. Shoji, N. Tanaka, K. Mikami, M. Uchiyama, T. Fukushima, Nat. Chem. 2014, 6, 498–503;
- 10bY. Shoji, N. Tanaka, D. Hashizume, T. Fukushima, Chem. Commun. 2015, 51, 13342–13345;
- 10cN. Tanaka, Y. Shoji, D. Hashizume, M. Sugimoto, T. Fukushima, Angew. Chem. Int. Ed. 2017, 56, 5312–5316; Angew. Chem. 2017, 129, 5396–5400.
- 11
- 11aK. Funahashi, N. Tanaka, Y. Shoji, N. Imazu, K. Nakayama, K. Kanahashi, H. Shirae, S. Noda, H. Ohta, T. Fukushima, T. Takenobu, Appl. Phys. Express 2017, 10, 035101;
- 11bH. Matsuoka, K. Kanahashi, N. Tanaka, Y. Shoji, L.-J. Li, J. Pu, H. Ito, T. Fukushima, T. Takenobu, Jpn. J. Appl. Phys. 2018, 57, 02CB15;
- 11cK. Kanahashi, N. Tanaka, Y. Shoji, M. Maruyama, I. Jeon, K. Kawahara, M. Ishihara, M. Hasegawa, H. Ohta, H. Ago, Y. Matsuo, S. Okada, T. Fukushima, T. Takenobu, npj 2D Mater. Appl. 2019, 3, 7;
- 11dB. Wegner, D. Lungwitz, A. E. Mansour, C. E. Tait, N. Tanaka, T. Zhai, S. Duhm, M. Forster, J. Behrends, Y. Shoji, A. Opitz, U. Scherf, E. J. W. List-Kratochvil, T. Fukushima, N. Koch, Adv. Sci. 2020, 7, 2001322.
- 12K. L. Bamford, Z.-W. Qu, D. W. Stephan, J. Am. Chem. Soc. 2019, 141, 6180–6184.
- 13See Supporting Information for experimental and computational details.
- 14
- 14aA. Berndt, H. Klusik, K. Schlüter, J. Organomet. Chem. 1981, 222, C25–C27;
- 14bH. Klusik, A. Berndt, Angew. Chem. Int. Ed. Engl. 1981, 20, 870–871; Angew. Chem. 1981, 93, 903–904;
- 14cW. J. Grigsby, P. P. Power, Chem. Commun. 1996, 2235–2236;
- 14dW. J. Grigsby, P. P. Power, Chem. Eur. J. 1997, 3, 368–375;
- 14eH. Asakawa, K.-H. Lee, K. Furukawa, Z. Lin, M. Yamashita, Chem. Eur. J. 2015, 21, 4267–4271.
- 15Deposition Numbers 2111231 (for Mes4B2) and 2111232 (for [Li+(DME)2THF][Mes4B2].−) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 16M. A. Haidekker, T. P. Brady, D. Lichlyter, E. A. Theodorakis, Bioorg. Chem. 2005, 33, 415–425.
- 17
- 17aZ. R. Grabowski, K. Rotkiewicz, W. Rettig, Chem. Rev. 2003, 103, 3899–4031;
- 17bS. Sasaki, G. P. C. Drummen, G. Konishi, J. Mater. Chem. C 2016, 4, 2731–2743.