Guest-Driven Light-Induced Spin Change in an Azobenzene Loaded Metal–Organic Framework
Kai-Ping Xie
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorZe-Yu Ruan
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorBang-Heng Lyu
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorXiao-Xian Chen
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorXue-Wen Zhang
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorGuo-Zhang Huang
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorDr. Yan-Cong Chen
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Zhao-Ping Ni
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Ming-Liang Tong
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorKai-Ping Xie
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorZe-Yu Ruan
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorBang-Heng Lyu
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorXiao-Xian Chen
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorXue-Wen Zhang
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorGuo-Zhang Huang
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorDr. Yan-Cong Chen
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Zhao-Ping Ni
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Ming-Liang Tong
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorGraphical Abstract
Abstract
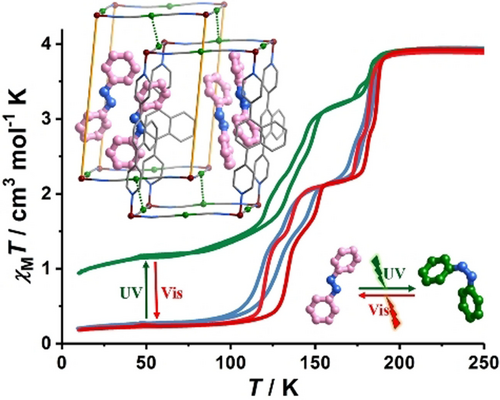
Stimuli-responsive materials that can be reversibly switched by light are of immense interest. Among them, photo-responsive spin crossover (SCO) complexes have great promises to combine the photoactive inputs with multifaceted outputs into switchable materials and devices. However, the reversible control the spin-state change by photochromic guests is still challenging. Herein, we report an unprecedented guest-driven light-induced spin change (GD-LISC) in a Hofmann-type metal–organic framework (MOF), [Fe(bpn){Ag(CN)2}2]⋅azobenzene. (1, bpn=1,4-bis(4-pyridyl)naphthalene). The reversible trans–cis photoisomerization of azobenzene guest upon UV/Vis irradiation in the solid-state results in the remarkable magnetic changes in a wide temperature range of 10–180 K. This finding not only establishes a new switching mechanism for SCO complexes, but also paves the way toward the development of new generation of photo-responsive magnetic materials.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202113294-sup-0001-cif.zip2.1 MB | Supporting Information |
| anie202113294-sup-0001-misc_information.pdf1.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. Yagai, A. Kitamura, Chem. Soc. Rev. 2008, 37, 1520–1529.
- 2S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan, A. L. Nussbaumer, Chem. Rev. 2015, 115, 10081–10206.
- 3W. Danowski, T. van Leeuwen, W. R. Browne, B. L. Feringa, Nanoscale Adv. 2021, 3, 24–40.
- 4S. Venkataramani, U. Jana, M. Dommaschk, F. D. Sönnichsen, F. Tuczek, R. Herges, Science 2011, 331, 445.
- 5M. Baroncini, S. d'Agostino, G. Bergamini, P. Ceroni, A. Comotti, P. Sozzani, I. Bassanetti, F. Grepioni, T. M. Hernandez, S. Silvi, M. Venturi, A. Credi, Nat. Chem. 2015, 7, 634–640.
- 6D. Samanta, J. Gemen, Z. Chu, Y. Diskin-Posner, L. J. W. Shimon, R. Klajn, Proc. Natl. Acad. Sci. USA 2018, 115, 9379.
- 7C. Park, K. Oh, S. C. Lee, C. Kim, Angew. Chem. Int. Ed. 2007, 46, 1455–1457; Angew. Chem. 2007, 119, 1477–1479.
- 8F. Muhammad, M. Guo, W. Qi, F. Sun, A. Wang, Y. Guo, G. Zhu, J. Am. Chem. Soc. 2011, 133, 8778–8781.
- 9P. K. Kundu, G. L. Olsen, V. Kiss, R. Klajn, Nat. Commun. 2014, 5, 3588.
- 10C. Gao, J. Li, S. Yin, J. Sun, C. Wang, Nat. Commun. 2020, 11, 4919.
- 11Z. Chu, Y. Han, T. Bian, S. De, P. Král, R. Klajn, J. Am. Chem. Soc. 2019, 141, 1949–1960.
- 12M. Vallet-Regi, A. Rámila, R. P. del Real, J. Pérez-Pariente, Chem. Mater. 2001, 13, 308–311.
- 13N. K. Mal, M. Fujiwara, Y. Tanaka, Nature 2003, 421, 350–353.
- 14Y. Ke, J. Chen, G. Lin, S. Wang, Y. Zhou, J. Yin, P. S. Lee, Y. Long, Adv. Energy Mater. 2019, 9, 1902066.
- 15R. D. Astumian, Chem. Sci. 2017, 8, 840–845.
- 16S.-L. Huang, T. S. A. Hor, G.-X. Jin, Coord. Chem. Rev. 2017, 346, 112–122.
- 17P. Howlader, B. Mondal, P. C. Purba, E. Zangrando, P. S. Mukherjee, J. Am. Chem. Soc. 2018, 140, 7952–7960.
- 18D. E. Williams, C. R. Martin, E. A. Dolgopolova, A. Swifton, D. C. Godfrey, O. A. Ejegbavwo, P. J. Pellechia, M. D. Smith, N. B. Shustova, J. Am. Chem. Soc. 2018, 140, 7611–7622.
- 19A. B. Grommet, L. M. Lee, R. Klajn, Acc. Chem. Res. 2020, 53, 2600–2610.
- 20A. B. Kanj, J. Bürck, N. Vankova, C. Li, D. Mutruc, A. Chandresh, S. Hecht, T. Heine, L. Heinke, J. Am. Chem. Soc. 2021, 143, 7059–7068.
- 21P. Gütlich, Y. Garcia, H. A. Goodwin, Chem. Soc. Rev. 2000, 29, 419–427.
- 22G. J. Halder, C. J. Kepert, B. Moubaraki, K. S. Murray, J. D. Cashion, Science 2002, 298, 1762.
- 23A. Bousseksou, G. Molnár, L. Salmon, W. Nicolazzi, Chem. Soc. Rev. 2011, 40, 3313–3335.
- 24S. Chorazy, T. Charytanowicz, D. Pinkowicz, J. Wang, K. Nakabayashi, S. Klimke, F. Renz, S.-i. Ohkoshi, B. Sieklucka, Angew. Chem. Int. Ed. 2020, 59, 15741–15749; Angew. Chem. 2020, 132, 15871–15879.
- 25M. A. Halcrow, Chem. Soc. Rev. 2011, 40, 4119–4142.
- 26M. C. Muñoz, J. A. Real, Coord. Chem. Rev. 2011, 255, 2068–2093.
- 27D. Aravena, E. Ruiz, J. Am. Chem. Soc. 2012, 134, 777–779.
- 28O. Sato, Nat. Chem. 2016, 8, 644–656.
- 29Y.-R. Qiu, L. Cui, P.-Y. Cai, F. Yu, M. Kurmoo, C. F. Leong, D. M. D'Alessandro, J.-L. Zuo, Chem. Sci. 2020, 11, 6229–6235.
- 30O. Kahn, C. J. Martinez, Science 1998, 279, 44.
- 31G. Molnár, S. Rat, L. Salmon, W. Nicolazzi, A. Bousseksou, Adv. Mater. 2018, 30, 1703862.
- 32R. Torres-Cavanillas, R. Sanchis-Gual, J. Dugay, M. Coronado-Puchau, M. Giménez-Marqués, E. Coronado, Adv. Mater. 2019, 31, 1900039.
- 33S. Decurtins, P. Gutlich, K. M. Hasselbach, A. Hauser, H. Spiering, Inorg. Chem. 1985, 24, 2174–2178.
- 34P. Poganiuch, S. Decurtins, P. Guetlich, J. Am. Chem. Soc. 1990, 112, 3270–3278.
- 35J.-F. Létard, L. Capes, G. Chastanet, N. Moliner, S. Létard, J.-A. Real, O. Kahn, Chem. Phys. Lett. 1999, 313, 115–120.
- 36J.-F. Létard, P. Guionneau, O. Nguyen, J. S. Costa, S. Marcén, G. Chastanet, M. Marchivie, L. Goux-Capes, Chem. Eur. J. 2005, 11, 4582–4589.
- 37M.-L. Boillot, S. Chantraine, J. Zarembowitch, J.-Y. Lallemand, J. Prunet, New J. Chem. 1999, 23, 179–184.
- 38“Spin Crossover in Transition Metal Compounds II”: M.-L. Boillot, J. Zarembowitch, A. Sour in Topics in Current Chemistry, Vol. 234 (Eds.: P. Gütlich, H. A. Goodwin), Springer, Heidelberg, 2004, pp. 261–276.
- 39M. Estrader, J. Salinas Uber, L. A. Barrios, J. Garcia, P. Lloyd-Williams, O. Roubeau, S. J. Teat, G. Aromí, Angew. Chem. Int. Ed. 2017, 56, 15622–15627; Angew. Chem. 2017, 129, 15828–15833.
- 40B. Doistau, L. Benda, B. Hasenknopf, V. Marvaud, G. Vives, Magnetochemistry 2018, 4, 5.
- 41B. Brachňaková, I. Šalitroš, Chem. Pap. 2018, 72, 773–798.
- 42Y. Hasegawa, S. Kume, H. Nishihara, Dalton Trans. 2009, 280–284.
- 43Y. Hasegawa, K. Takahashi, S. Kume, H. Nishihara, Chem. Commun. 2011, 47, 6846–6848.
- 44K. Takahashi, Y. Hasegawa, R. Sakamoto, M. Nishikawa, S. Kume, E. Nishibori, H. Nishihara, Inorg. Chem. 2012, 51, 5188–5198.
- 45S. O. Schmidt, H. Naggert, A. Buchholz, H. Brandenburg, A. Bannwarth, W. Plass, F. Tuczek, Eur. J. Inorg. Chem. 2016, 2175–2186.
- 46M. Milek, F. W. Heinemann, M. M. Khusniyarov, Inorg. Chem. 2013, 52, 11585–11592.
- 47B. Rösner, M. Milek, A. Witt, B. Gobaut, P. Torelli, R. H. Fink, M. M. Khusniyarov, Angew. Chem. Int. Ed. 2015, 54, 12976–12980; Angew. Chem. 2015, 127, 13168–13172.
- 48H. M. D. Bandara, S. C. Burdette, Chem. Soc. Rev. 2012, 41, 1809–1825.
- 49N. Yanai, T. Uemura, M. Inoue, R. Matsuda, T. Fukushima, M. Tsujimoto, S. Isoda, S. Kitagawa, J. Am. Chem. Soc. 2012, 134, 4501–4504.
- 50A. Knebel, L. Sundermann, A. Mohmeyer, I. Strauß, S. Friebe, P. Behrens, J. Caro, Chem. Mater. 2017, 29, 3111–3117.
- 51J. He, K. Aggarwal, N. Katyal, S. He, E. Chiang, S. G. Dunning, J. E. Reynolds, A. Steiner, G. Henkelman, E. L. Que, S. M. Humphrey, J. Am. Chem. Soc. 2020, 142, 6467–6471.
- 52M. Ohba, K. Yoneda, G. Agustí, M. C. Muñoz, A. B. Gaspar, J. A. Real, M. Yamasaki, H. Ando, Y. Nakao, S. Sakaki, S. Kitagawa, Angew. Chem. Int. Ed. 2009, 48, 4767–4771; Angew. Chem. 2009, 121, 4861–4865.
- 53Z.-P. Ni, J.-L. Liu, M. N. Hoque, W. Liu, J.-Y. Li, Y.-C. Chen, M.-L. Tong, Coord. Chem. Rev. 2017, 335, 28–43.
- 54M. Feng, Z.-Y. Ruan, Y.-C. Chen, M.-L. Tong, Chem. Commun. 2020, 56, 13702–13718.
- 55T. Delgado, M. Meneses-Sánchez, L. Piñeiro-López, C. Bartual-Murgui, M. C. Muñoz, J. A. Real, Chem. Sci. 2018, 9, 8446–8452.
- 56L. Piñeiro-López, F.-J. Valverde-Muñoz, E. Trzop, M. C. Muñoz, M. Seredyuk, J. Castells-Gil, I. da Silva, C. Martí-Gastaldo, E. Collet, J. A. Real, Chem. Sci. 2021, 12, 1317–1326.
- 57X. Bao, H. J. Shepherd, L. Salmon, G. Molnár, M.-L. Tong, A. Bousseksou, Angew. Chem. Int. Ed. 2013, 52, 1198–1202; Angew. Chem. 2013, 125, 1236–1240.
- 58B. Brachňaková, J. Moncoľ, J. Pavlik, I. Šalitroš, S. Bonhommeau, F. J. Valverde-Muñoz, L. Salmon, G. Molnár, L. Routaboul, A. Bousseksou, Dalton Trans. 2021, 50, 8877–8888.
- 59P. Gütlich, A. Hauser, H. Spiering, Angew. Chem. Int. Ed. Engl. 1994, 33, 2024–2054; Angew. Chem. 1994, 106, 2109–2141.
- 60E. Milin, V. Patinec, S. Triki, E.-E. Bendeif, S. Pillet, M. Marchivie, G. Chastanet, K. Boukheddaden, Inorg. Chem. 2016, 55, 11652–11661.
- 61T. Boonprab, S. J. Lee, S. G. Telfer, K. S. Murray, W. Phonsri, G. Chastanet, E. Collet, E. Trzop, G. N. L. Jameson, P. Harding, D. J. Harding, Angew. Chem. Int. Ed. 2019, 58, 11811–11815; Angew. Chem. 2019, 131, 11937–11941.
- 62Y.-C. Chen, Y. Meng, Y.-J. Dong, X.-W. Song, G.-Z. Huang, C.-L. Zhang, Z.-P. Ni, J. Navařík, O. Malina, R. Zbořil, M.-L. Tong, Chem. Sci. 2020, 11, 3281–3289.
- 63Deposition Numbers 2093241, 2093242, 2093243, 2093244, and 2093245 contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 64M. Paez-Espejo, M. Sy, K. Boukheddaden, J. Am. Chem. Soc. 2016, 138, 3202–3210.
- 65L. Duarte, R. Fausto, I. Reva, Phys. Chem. Chem. Phys. 2014, 16, 16919–16930.
- 66Z. Wang, A. Knebel, S. Grosjean, D. Wagner, S. Bräse, C. Wöll, J. Caro, L. Heinke, Nat. Commun. 2016, 7, 13872.
- 67D. Hermann, H. Emerich, R. Lepski, D. Schaniel, U. Ruschewitz, Inorg. Chem. 2013, 52, 2744–2749.
- 68P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka, M. A. Spackman, J. Appl. Crystallogr. 2021, 54, 1006–1011.