Engineering Lattice Oxygen Activation of Iridium Clusters Stabilized on Amorphous Bimetal Borides Array for Oxygen Evolution Reaction
Chen Wang
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorPanlong Zhai
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorMingyue Xia
Laboratory of Materials Modification by Laser, Ion and Electron Beams, Ministry of Education, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorYunzhen Wu
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorBo Zhang
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorZhuwei Li
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorDr. Lei Ran
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Junfeng Gao
Laboratory of Materials Modification by Laser, Ion and Electron Beams, Ministry of Education, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorXiaomeng Zhang
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorZhaozhong Fan
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorProf. Dr. Licheng Sun
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Center of Artificial Photosynthesis for Solar Fuels, School of Science, Westlake University, Hangzhou, 310024 P. R. China
School of Engineering Sciences in Chemistry, Biotechnology and Health, KTH Royal Institute of Technology, 10044 Stockholm, Sweden
Search for more papers by this authorCorresponding Author
Prof. Dr. Jungang Hou
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorChen Wang
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorPanlong Zhai
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorMingyue Xia
Laboratory of Materials Modification by Laser, Ion and Electron Beams, Ministry of Education, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorYunzhen Wu
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorBo Zhang
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorZhuwei Li
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorDr. Lei Ran
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Junfeng Gao
Laboratory of Materials Modification by Laser, Ion and Electron Beams, Ministry of Education, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorXiaomeng Zhang
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorZhaozhong Fan
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorProf. Dr. Licheng Sun
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Center of Artificial Photosynthesis for Solar Fuels, School of Science, Westlake University, Hangzhou, 310024 P. R. China
School of Engineering Sciences in Chemistry, Biotechnology and Health, KTH Royal Institute of Technology, 10044 Stockholm, Sweden
Search for more papers by this authorCorresponding Author
Prof. Dr. Jungang Hou
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, 2, Linggong Road, Dalian, 116024 P. R. China
Search for more papers by this authorGraphical Abstract
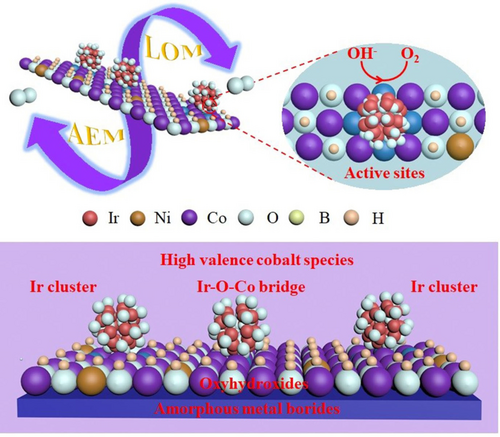
Iridium clusters stabilized surface reconstructed oxyhydroxides on an amorphous metal boride array are reported, achieving an ultralow overpotential of 178 mV at 10 mA cm−2 for OER in alkaline medium. The coupling of iridium clusters induces the formation of high-valence cobalt species and Ir–O–Co bridges between iridium and oxyhydroxides at the atomic scale.
Abstract
Developing robust oxygen evolution reaction (OER) catalysts requires significant advances in material design and in-depth understanding for water electrolysis. Herein, we report iridium clusters stabilized surface reconstructed oxyhydroxides on amorphous metal borides array, achieving an ultralow overpotential of 178 mV at 10 mA cm−2 for OER in alkaline medium. The coupling of iridium clusters induced the formation of high valence cobalt species and Ir–O–Co bridge between iridium and oxyhydroxides at the atomic scale, engineering lattice oxygen activation and non-concerted proton-electron transfer to trigger multiple active sites for intrinsic pH-dependent OER activity. The lattice oxygen oxidation mechanism (LOM) was confirmed by in situ 18O isotope labeling mass spectrometry and chemical recognition of negative peroxo-like species. Theoretical simulations reveal that the OER performance on this catalyst is intrinsically dominated by LOM pathway, facilitating the reaction kinetics. This work not only paves an avenue for the rational design of electrocatalysts, but also serves the fundamental insights into the lattice oxygen participation for promising OER application.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202112870-sup-0001-misc_information.pdf3.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. A. Turner, Science 2004, 305, 972–974.
- 2Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Norskov, T. F. Jaramillo, Science 2017, 355, eaad4998.
- 3S. Chu, A. Majumdar, Nature 2012, 488, 294–303.
- 4X. Zou, Y. Zhang, Chem. Soc. Rev. 2015, 44, 5148–5180.
- 5J. Song, C. Wei, Z. F. Huang, C. Liu, L. Zeng, X. Wang, Z. J. Xu, Chem. Soc. Rev. 2020, 49, 2196–2214.
- 6B. M. Hunter, H. B. Gray, A. M. Muller, Chem. Rev. 2016, 116, 14120–14136.
- 7Y. Sun, H. Liao, J. Wang, B. Chen, S. Sun, S. J. H. Ong, S. Xi, C. Diao, Y. Du, J. O. Wang, M. B. H. Breese, S. Li, H. Zhang, Z. J. Xu, Nat. Catal. 2020, 3, 554–563.
- 8H. Wang, J. Wang, Y. Pi, Q. Shao, Y. Tan, X. Huang, Angew. Chem. Int. Ed. 2019, 58, 2316–2320; Angew. Chem. 2019, 131, 2338–2342.
- 9A. Grimaud, O. Diaz-Morales, B. Han, W. T. Hong, Y. L. Lee, L. Giordano, K. A. Stoerzinger, M. T. M. Koper, Y. Shao-Horn, Nat. Chem. 2017, 9, 457–465.
- 10M. Chen, Y. Xie, J. X. Wu, H. Huang, J. Teng, D. Wang, Y. Fan, J. J. Jiang, H. P. Wang, C. Y. Su, J. Mater. Chem. A 2019, 7, 10217–10224.
- 11P. W. Menezes, A. Indra, C. Das, C. Walter, C. Göbel, V. Gutkin, D. Schmeiβer, M. Driess, ACS Catal. 2017, 7, 103–109.
- 12J. Yin, J. Jin, H. Lin, Z. Yin, J. Li, M. Lu, L. Guo, P. Xi, Y. Tang, C. H. Yan, Adv. Sci. 2020, 7, 1903070.
- 13Y. Hou, M. Qiu, M. G. Kim, P. Liu, G. Nam, T. Zhang, X. Zhuang, B. Yang, J. Cho, M. Chen, C. Yuan, L. Lei, X. Feng, Nat. Commun. 2019, 10, 1392.
- 14Y. R. Hong, K. M. Kim, J. H. Ryu, S. Mhin, J. Kim, G. Ali, K. Y. Chung, S. Kang, H. Han, Adv. Funct. Mater. 2020, 30, 2004330.
- 15T. Tan, P. Han, H. Cong, G. Cheng, W. Luo, ACS Sustainable Chem. Eng. 2019, 7, 5620–5625.
- 16M. Kim, B. Lee, H. Ju, S. W. Lee, J. Kim, Adv. Mater. 2019, 31, 1901977.
- 17D. Liu, H. Ai, J. Li, M. Fang, M. Chen, D. Liu, X. Du, P. Zhou, F. Li, K. H. Lo, Y. Tang, S. Chen, L. Wang, G. Xing, H. Pan, Adv. Energy Mater. 2020, 10, 2002464.
- 18Y. Duan, Z. Y. Yu, S. J. Hu, X. S. Zheng, C. T. Zhang, H. H. Ding, B. C. Hu, Q. Q. Fu, Z. L. Yu, X. Zheng, J. F. Zhu, M. R. Gao, S. H. Yu, Angew. Chem. Int. Ed. 2019, 58, 15772–15777; Angew. Chem. 2019, 131, 15919–15924.
- 19J. Wang, S. J. Kim, J. Liu, Y. Gao, S. Choi, J. Han, H. Shin, S. Jo, J. Kim, F. Ciucci, H. Kim, Q. Li, W. Yang, X. Long, S. Yang, S.-P. Cho, K. H. Chae, M. G. Kim, H. Kim, J. Lim, Nat. Catal. 2021, 4, 212–222.
- 20E. Fabbri, M. Nachtegaal, T. Binninger, X. Cheng, B. J. Kim, J. Durst, F. Bozza, T. Graule, R. Schaublin, L. Wiles, M. Pertoso, N. Danilovic, K. E. Ayers, T. J. Schmidt, Nat. Mater. 2017, 16, 925–931.
- 21C. Baeumer, J. Li, Q. Lu, A. Y. Liang, L. Jin, H. P. Martins, T. Duchon, M. Gloss, S. M. Gericke, M. A. Wohlgemuth, M. Giesen, E. E. Penn, R. Dittmann, F. Gunkel, R. Waser, M. Bajdich, S. Nemsak, J. T. Mefford, W. C. Chueh, Nat. Mater. 2021, 20, 674–682.
- 22Z. Ma, Y. Zhang, S. Liu, W. Xu, L. Wu, Y. C. Hsieh, P. Liu, Y. Zhu, K. Sasaki, J. N. Renner, K. E. Ayers, R. R. Adzic, J. X. Wang, J. Electroanal. Chem. 2018, 819, 296–305.
- 23Y. Peng, Q. Liu, B. Lu, T. He, F. Nichols, X. Hu, T. Huang, G. Huang, L. Guzman, Y. Ping, S. Chen, ACS Catal. 2021, 11, 1179–1188.
- 24H. Hu, F. M. D. Kazim, Z. Ye, Y. Xie, Q. Zhang, K. Qu, J. Xu, W. Cai, S. Xiao, Z. Yang, J. Mater. Chem. A 2020, 8, 20168–20174.
- 25C. Cai, M. Wang, S. Han, Q. Wang, Q. Zhang, Y. Zhu, X. Yang, D. Wu, X. Zu, G. E. Sterbinsky, Z. Feng, M. Gu, ACS Catal. 2021, 11, 123–130.
- 26K. Jiang, M. Luo, M. Peng, Y. Yu, Y. R. Lu, T. S. Chan, P. Liu, F. M. F. de Groot, Y. Tan, Nat. Commun. 2020, 11, 2701.
- 27S. Gupta, M. K. Patel, A. Miotello, N. Patel, Adv. Funct. Mater. 2020, 30, 1906481.
- 28J. T. Mefford, X. Rong, A. M. Abakumov, W. G. Hardin, S. Dai, A. M. Kolpak, K. P. Johnston, K. J. Stevenson, Nat. Commun. 2016, 7, 11053.
- 29Y. Surendranath, M. W. Kanan, D. G. Nocera, J. Am. Chem. Soc. 2010, 132, 16501–16509.
- 30Z. F. Huang, J. Song, Y. Du, S. Xi, S. Dou, J. M. V. Nsanzimana, C. Wang, Z. J. Xu, X. Wang, Nat. Energy 2019, 4, 329–338.
- 31P. Zhang, L. Li, D. Nordlund, H. Chen, L. Fan, B. Zhang, X. Sheng, Q. Daniel, L. Sun, Nat. Commun. 2018, 9, 381.
- 32Y. Pan, X. Xu, Y. Zhong, L. Ge, Y. Chen, J. M. Veder, D. Guan, R. O'Hayre, M. Li, G. Wang, H. Wang, W. Zhou, Z. Shao, Nat. Commun. 2020, 11, 2002.
- 33G. Zhao, P. Li, N. Cheng, S. X. Dou, W. Sun, Adv. Mater. 2020, 32, 2000872.
- 34N. Xu, G. Cao, Z. Chen, Q. Kang, H. Dai, P. Wang, J. Mater. Chem. A 2017, 5, 12379–12384.
- 35S. H. Ye, Z. X. Shi, J. X. Feng, Y. X. Tong, G. R. Li, Angew. Chem. Int. Ed. 2018, 57, 2672–2676; Angew. Chem. 2018, 130, 2702–2706.
- 36H. Sun, L. Chen, Y. Lian, W. Yang, L. Lin, Y. Chen, J. Xu, D. Wang, X. Yang, M. H. Rummerli, J. Guo, J. Zhong, Z. Deng, Y. Jiao, Y. Peng, S. Qiao, Adv. Mater. 2020, 32, 2006784.
- 37H. S. Oh, H. N. Nong, T. Reier, A. Bergmann, M. Gliech, J. Ferreira de Araujo, E. Willinger, R. Schlögl, D. Teschner, P. Strasser, J. Am. Chem. Soc. 2016, 138, 12552–12563.
- 38J. Zhang, J. Liu, L. Xi, Y. Yu, N. Chen, S. Sun, W. Wang, K. M. Lange, B. Zhang, J. Am. Chem. Soc. 2018, 140, 3876–3879.
- 39Y. Sun, K. Xu, Z. Wei, H. Li, T. Zhang, X. Li, W. Cai, J. Ma, H. J. Fan, Y. Li, Adv. Mater. 2018, 30, 1802121.
- 40J. X. Feng, S. H. Ye, H. Xu, Y. X. Tong, G. R. Li, Adv. Mater. 2016, 28, 4698–4703.
- 41W. Wang, Y. Jiang, Y. Hu, Y. Liu, J. Li, S. Chen, ACS Appl. Mater. Interfaces 2020, 12, 11600–11606.
- 42Y. P. Zhu, T. Y. Ma, M. Jaroniec, S. Z. Qiao, Angew. Chem. Int. Ed. 2017, 56, 1324–1328; Angew. Chem. 2017, 129, 1344–1348.
- 43H. Zhang, X. Li, A. Hähnel, V. Naumann, C. Lin, S. Azimi, S. L. Schweizer, A. W. Maijenburg, R. B. Wehrspohn, Adv. Funct. Mater. 2018, 28, 1706847.
- 44Y. Lu, C. Li, Y. Zhang, X. Cao, G. Xie, M. Wang, D. Peng, K. Huang, B. Zhang, T. Wang, W. Junsheng, Y. Huang, Nano Energy 2021, 83, 105800.
- 45P. W. Menezes, C. Walter, B. Chakraborty, J. N. Hausmann, I. Zaharieva, A. Frick, E. von Hauff, H. Dau, M. Driess, Adv. Mater. 2021, 33, 2004098.
- 46D. Mullangi, V. Dhavale, S. Shalini, S. Nandi, S. Collins, T. Woo, S. Kurungot, R. Vaidhyanathan, Adv. Energy Mater. 2016, 6, 1600110.
- 47J. W. D. Ng, M. García-Melchor, M. Bajdich, P. Chakthranont, C. Kirk, A. Vojvodic, T. F. Jaramillo, Nat. Energy 2016, 1, 16053.
- 48N. W. Yang, D. Chen, P. Cui, T. Lu, H. Liu, C. Hu, L. Xu, J. Yang, SmartMat 2021, 2, 234–245.
- 49X. Ding, H. Huang, Q. Wan, X. Guan, Y. Fang, S. Lin, D. Chen, Z. Xie, J. Energy Chem. 2021, 62, 415–422
- 50X. Wang, T. Ouyang, L. Wang, J. Zhong, Z. Liu, Angew. Chem. Int. Ed. 2020, 59, 6492–6499; Angew. Chem. 2020, 132, 6554–6561.
- 51J. Wang, K. Li, H. X. Zhong, D. Xu, Z. L. Wang, Z. Jiang, Z. J. Wu, X. B. Zhang, Angew. Chem. Int. Ed. 2015, 54, 10530–10534; Angew. Chem. 2015, 127, 10676–10680.
- 52X. Li, L. Xiao, L. Zhou, Q. Xu, J. Weng, J. Xu, B. Liu, Angew. Chem. Int. Ed. 2020, 59, 21106–21113; Angew. Chem. 2020, 132, 21292–21299.
- 53W. Huang, C. Peng, J. Tang, F. Diao, M. Yesibolati, H. Sun, C. Engelbrekt, J. Zhang, X. Xiao, K. S. Mølhave, J. Energy Chem. 2022, 65, 78–88
- 54Q. Wang, L. Shang, X. Sun-Waterhouse, T. Zhang, G. Waterhouse, SmartMat 2021, 2, 154–175.
- 55T. Ouyang, X. Wang, X. Mai, A. Chen, Z. Tang, Z. Liu, Angew. Chem. Int. Ed. 2020, 59, 11948–11957; Angew. Chem. 2020, 132, 12046–12055.