Five-Fold Symmetric Pentaindolo- and Pentakis(benzoindolo)Corannulenes: Unique Structural Dynamics Derived from the Combination of Helical and Bowl Inversions
Koki Kise
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorDr. Shota Ooi
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorProf. Dr. Hayate Saito
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorProf. Dr. Hideki Yorimitsu
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorProf. Dr. Atsuhiro Osuka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Takayuki Tanaka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorKoki Kise
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorDr. Shota Ooi
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorProf. Dr. Hayate Saito
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorProf. Dr. Hideki Yorimitsu
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorProf. Dr. Atsuhiro Osuka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Takayuki Tanaka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorGraphical Abstract
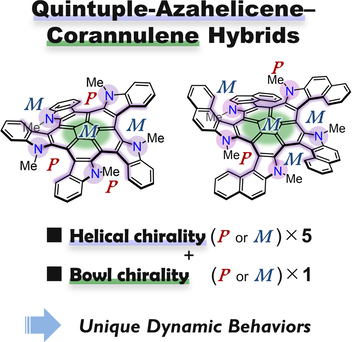
Peripherally π-extended corannulenes with five azahelicene units were prepared and their structural dynamics were studied experimentally and theoretically. This motif contains many conformational isomers owing to the helical and bowl chiralities. The interconversion networks were explored by using GRRM17 program, which revealed that the co-existing corannulene and azahelicene moieties lower the activation energy barriers for some isomerization processes.
Abstract
Peripherally π-extended corannulenes bearing quintuple azahelicene units, 10 and 11, were prepared and their dynamic behaviors were studied experimentally and theoretically. The fused corannulenes were synthesized from sym-pentabromocorannulene in three steps. X-Ray diffraction analysis for 10 displayed a conformer possessing a P(M) bowl chirality and a PPMPM (PMPMM) helical chirality, which was found to be the most stable conformer(s). Variable-temperature NMR measurements of 10 and 11 revealed that their structural isomers can be interconvertible in solution, depending on the steric congestion around the helical scaffolds. Automated search for conformers in the equilibrium and transition states by Artificial Force Induced Reaction (AFIR) method revealed their interconversion networks, including bowl-inversion and helical-inversion. This analysis indicated that the co-existing corannulene and azahelicene moieties influence the conformational dynamics, which leads to mitigation of the activation energy barriers for isomerization.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202112589-sup-0001-cif.zip1.4 MB | Supporting Information |
| anie202112589-sup-0001-misc_information.pdf18.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aW. E. Barth, R. G. Lawton, J. Am. Chem. Soc. 1966, 88, 380;
- 1bV. M. Tsefrikas, L. T. Scott, Chem. Rev. 2006, 106, 4868;
- 1cA. M. Butterfield, B. Gilomen, J. S. Siegel, Org. Process Res. Dev. 2012, 16, 664;
- 1dX. Li, F. Kang, M. Inagaki, Small 2016, 12, 3206;
- 1eE. Nestoros, M. C. Stuparu, Chem. Commun. 2018, 54, 6503;
- 1fE. M. Muzammil, D. Halilovic, M. C. Stuparu, Commun. Chem. 2019, 2, 58;
- 1gM. C. Stuparu, Acc. Chem. Res. 2021, 54, 2858.
- 2
- 2aD. Miyajima, K. Tashiro, F. Araoka, H. Takezoe, J. Kim, K. Kato, M. Takata, T. Aida, J. Am. Chem. Soc. 2009, 131, 44;
- 2bM. C. Stuparu, J. Polym. Sci. Part A 2012, 50, 2641;
- 2cJ. Kang, D. Miyajima, Y. Itoh, T. Mori, H. Tanaka, M. Yamauchi, Y. Inoue, S. Harada, T. Aida, J. Am. Chem. Soc. 2014, 136, 10640;
- 2dJ. Kang, D. Miyajima, T. Mori, Y. Inoue, Y. Itoh, T. Aida, Science 2015, 347, 646;
- 2eS. H. Mahadevegowda, M. C. Stuparu, ACS Omega 2017, 2, 4964;
- 2fF. Huang, L. Ma, Y. Che, H. Jiang, X. Chen, Y. Wang, J. Org. Chem. 2018, 83, 733.
- 3
- 3aK. Shi, T. Lei, X.-Y. Wang, J.-Y. Wang, J. Pei, Chem. Sci. 2014, 5, 1041;
- 3bR.-Q. Lu, Y.-N. Zhou, X.-Y. Yan, K. Shi, Y.-Q. Zheng, M. Luo, X.-C. Wang, J. Pei, H. Xia, L. Zoppi, K. K. Baldridge, J. S. Siegel, X.-Y. Cao, Chem. Commun. 2015, 51, 1681;
- 3cR. Chen, R.-Q. Lu, K. Shi, F. Wu, H.-X. Fang, Z.-X. Niu, X.-Y. Yan, M. Luo, X.-C. Wang, C.-Y. Yang, X.-Y. Wang, B. Xu, H. Xia, J. Pei, X.-Y. Cao, Chem. Commun. 2015, 51, 13768.
- 4
- 4aJ. Mack, P. Vogel, D. Jones, N. Kaval, A. Sutton, Org. Biomol. Chem. 2007, 5, 2448;
- 4bG. Valenti, C. Bruno, S. Rapino, A. Fiorani, E. A. Jackson, L. T. Scott, F. Paolucci, M. Marcaccio, J. Phys. Chem. C 2010, 114, 19467;
- 4cJ. Li, A. Terec, Y. Wang, H. Joshi, Y. Lu, H. Sun, M. C. Stuparu, J. Am. Chem. Soc. 2017, 139, 3089.
- 5W. B. Fellows, A. M. Rice, D. E. Williams, E. A. Dolgopolova, A. K. Vannucci, P. J. Pellechia, M. D. Smith, J. A. Krause, N. B. Shustova, Angew. Chem. Int. Ed. 2016, 55, 2195; Angew. Chem. 2016, 128, 2235.
- 6
- 6aL. T. Scott, E. A. Jackson, Q. Zhang, B. D. Steinberg, M. Bancu, B. Li, J. Am. Chem. Soc. 2012, 134, 107;
- 6bK. Kawasumi, Q. Zhang, Y. Segawa, L. T. Scott, K. Itami, Nat. Chem. 2013, 5, 739;
- 6cK. Kato, K. Takaba, S. Maki-Yonekura, N. Mitoma, Y. Nakanishi, T. Nishihara, T. Hatakeyama, T. Kawada, Y. Hijikata, J. Pirillo, L. T. Scott, K. Yonekura, Y. Segawa, K. Itami, J. Am. Chem. Soc. 2021, 143, 5465.
- 7
- 7aH. Yokoi, Y. Hiraoka, S. Hiroto, D. Sakamaki, S. Seki, H. Shinokubo, Nat. Commun. 2015, 6, 8215;
- 7bS. Ito, Y. Tokimaru, K. Nozaki, Angew. Chem. Int. Ed. 2015, 54, 7256; Angew. Chem. 2015, 127, 7364;
- 7cV. M. Tsefrikas, A. K. Greene, L. T. Scott, Org. Chem. Front. 2017, 4, 688;
- 7dT. Nagano, K. Nakamura, Y. Tokimaru, S. Ito, D. Miyajima, T. Aida, K. Nozaki, Chem. Eur. J. 2018, 24, 14075;
- 7eH. Yokoi, S. Hiroto, D. Sakamaki, S. Seki, H. Shinokubo, Chem. Sci. 2018, 9, 819;
- 7fH. Yokoi, S. Hiroto, H. Shinokubo, J. Am. Chem. Soc. 2018, 140, 4649;
- 7gM. Takeda, S. Hiroto, H. Yokoi, S. Lee, D. Kim, H. Shinokubo, J. Am. Chem. Soc. 2018, 140, 6336;
- 7hZ. Zhou, Z. Wei, Y. Tokimaru, S. Ito, K. Nozaki, M. A. Petrukhina, Angew. Chem. Int. Ed. 2019, 58, 12107; Angew. Chem. 2019, 131, 12235.
- 8
- 8aA. Sygula, R. Sygula, P. W. Rabideau, Org. Lett. 2005, 7, 4999;
- 8bM. C. Stuparu, Tetrahedron 2012, 68, 3527;
- 8cN. Niamnont, N. Kimpitak, K. Wongravee, P. Rashatasakhon, K. K. Baldridge, J. S. Siegel, M. Sukwattanasinitt, Chem. Commun. 2013, 49, 780;
- 8dY. Tokimaru, S. Ito, K. Nozaki, Angew. Chem. Int. Ed. 2017, 56, 15560; Angew. Chem. 2017, 129, 15766;
- 8eC. Mejuto, L. Escobar, G. Guisado-Barri, P. Ballester, D. Gusev, E. Peris, Chem. Eur. J. 2017, 23, 10644;
- 8fY. Wang, O. Allemann, T. S. Balaban, N. Vanthuyne, A. Linden, K. K. Baldridge, J. S. Siegel, Angew. Chem. Int. Ed. 2018, 57, 6470; Angew. Chem. 2018, 130, 6580;
- 8gY. Tokimaru, S. Ito, K. Nozaki, Angew. Chem. Int. Ed. 2018, 57, 9818; Angew. Chem. 2018, 130, 9966;
- 8hY.-Y. Xu, H.-R. Tian, S.-H. Li, Z.-C. Chen, Y.-R. Yao, S.-S. Wang, X. Zhang, Z.-Z. Zhu, S.-L. Deng, Q. Zhang, S. Yang, S.-Y. Xie, R.-B. Huang, L.-S. Zheng, Nat. Commun. 2019, 10, 485;
- 8iF. Xie, N. S. Finney, Chem. Commun. 2020, 56, 10525;
- 8jA. Ghosh, D. Csókás, M. Budanović, R. D. Webster, I. Pápai, M. C. Stuparu, Chem. Sci. 2021, 12, 3977.
- 9Recent reviews on helicenes:
- 9aY. Shen, C.-F. Chen, Chem. Rev. 2012, 112, 1463;
- 9bM. Gingras, Chem. Soc. Rev. 2013, 42, 968;
- 9cW.-L. Zhao, M. Li, H.-Y. Lu, C.-F. Chen, Chem. Commun. 2019, 55, 13793;
- 9dK. Dhbaibi, L. Favereau, J. Crassous, Chem. Rev. 2019, 119, 8846;
- 9eI. G. Stará, I. Starý, Acc. Chem. Res. 2020, 53, 144;
- 9fK. M. Magiera, V. Aryal, W. A. Chalifoux, Org. Biomol. Chem. 2020, 18, 2372;
- 9gT. Mori, Chem. Rev. 2021, 121, 2373.
- 10
- 10aM. Yanney, F. R. Fronczek, W. P. Henry, D. J. Beard, A. Sygula, Eur. J. Org. Chem. 2011, 6636;
- 10bT. Fujikawa, D. V. Preda, Y. Segawa, K. Itami, L. T. Scott, Org. Lett. 2016, 18, 3992;
- 10cK. Kato, Y. Segawa, L. T. Scott, K. Itami, Angew. Chem. Int. Ed. 2018, 57, 1337; Angew. Chem. 2018, 130, 1351;
- 10dH.-A. Lin, K. Kato, Y. Segawa, L. T. Scott, K. Itami, Chem. Sci. 2019, 10, 2326;
- 10eD. Meng, G. Liu, C. Xiao, Y. Shi, L. Zhang, L. Jiang, K. K. Baldridge, Y. Li, J. S. Siegel, Z. Wang, J. Am. Chem. Soc. 2019, 141, 5402;
- 10fS. Chen, D. Meng, J. Huang, N. Liang, Y. Li, F. Liu, H. Yan, Z. Wang, CCS Chem. 2021, 3, 78.
- 11
- 11aL. Barnett, D. M. Ho, K. K. Baldridge, R. A. Pascal, Jr., J. Am. Chem. Soc. 1999, 121, 727;
- 11bA. Pradhan, P. Dechambenoit, H. Bock, F. Durola, Angew. Chem. Int. Ed. 2011, 50, 12582; Angew. Chem. 2011, 123, 12790;
- 11cJ. Luo, X. Xu, R. Mao, Q. Miao, J. Am. Chem. Soc. 2012, 134, 13796;
- 11dT. Fujikawa, Y. Segawa, K. Itami, J. Am. Chem. Soc. 2015, 137, 7763;
- 11eD. Meng, H. Fu, C. Xiao, X. Meng, T. Winands, W. Ma, W. Wei, B. Fan, L. Huo, N. L. Doltsinis, Y. Li, Y. Sun, Z. Wang, J. Am. Chem. Soc. 2016, 138, 10184;
- 11fY. Hu, X.-Y. Wang, P.-X. Peng, X.-C. Wang, X.-Y. Cao, X. Feng, K. Mgllen, A. Narita, Angew. Chem. Int. Ed. 2017, 56, 3374; Angew. Chem. 2017, 129, 3423;
- 11gM. Ferreira, G. Naulet, H. Gallardo, P. Dechambenoit, H. Bock, F. Durola, Angew. Chem. Int. Ed. 2017, 56, 3379; Angew. Chem. 2017, 129, 3428;
- 11hV. Berezhnaia, M. Roy, N. Vanthuyne, M. Villa, J.-V. Naubron, J. Rodriguez, Y. Coquerel, M. Gingras, J. Am. Chem. Soc. 2017, 139, 18508;
- 11iT. Hosokawa, Y. Takahashi, T. Matsushima, S. Watanabe, S. Kikkawa, I. Azumaya, A. Tsurusaki, K. Kamikawa, J. Am. Chem. Soc. 2017, 139, 18512;
- 11jY. Hu, G. M. Paternò, X.-Y. Wang, X.-C. Wang, M. Guizzardi, Q. Chen, D. Schollmeyer, X.-Y. Cao, G. Cerullo, F. Scotognella, K. Müllen, A. Narita, J. Am. Chem. Soc. 2019, 141, 12797;
- 11kA. E. Samkian, G. R. Kiel, C. G. Jones, H. M. Bergman, J. Oktawiec, H. M. Nelson, T. D. Tilley, Angew. Chem. Int. Ed. 2021, 60, 2493; Angew. Chem. 2021, 133, 2523;
- 11lF. Zhou, Z. Huang, Z. Huang, R. Cheng, Y. Yang, J. You, Org. Lett. 2021, 23, 4559.
- 12K. Kise, S. Ooi, A. Osuka, T. Tanaka, Asian J. Org. Chem. 2021, 10, 537.
- 13B. Topolinski, B. M. Schmidt, M. Kathan, S. I. Troyanov, D. Lentz, Chem. Commun. 2012, 48, 6298.
- 14It has been reported in similar transformations that the reactions under microwave heating were much more reproducible than the same reactions under conventional heating:
- 14aE. A. Jackson, B. D. Steinberg, M. Bancu, A. Wakamiya, L. T. Scott, J. Am. Chem. Soc. 2007, 129, 484;
- 14bB. D. Steinberg, E. A. Jackson, A. S. Filatov, A. Wakamiya, M. A. Petrukhina, L. T. Scott, J. Am. Chem. Soc. 2009, 131, 10537.
- 15
- 15aL. T. Scott, M. M. Hashemi, M. S. Bratcher, J. Am. Chem. Soc. 1992, 114, 1920;
- 15bT. J. Seiders, K. K. Baldridge, G. H. Grube, J. S. Siegel, J. Am. Chem. Soc. 2001, 123, 517.
- 16Scarce examples specifically focusing on the fluorescence properties of corannulenes: ref. [4c] and
- 16aY.-T. Wu, D. Bandera, R. Maag, A. Linden, K. K. Baldridge, J. S. Siegel, J. Am. Chem. Soc. 2008, 130, 10729;
- 16bY.-L. Wu, M. C. Stuparu, C. Boudon, J.-P. Gisselbrecht, W. B. Schweizer, K. K. Baldridge, J. S. Siegel, F. Diederich, J. Org. Chem. 2012, 77, 11014;
- 16cA. K. Dutta, A. Linden, L. Zoppi, K. K. Baldridge, J. S. Siegel, Angew. Chem. Int. Ed. 2015, 54, 10792; Angew. Chem. 2015, 127, 10942.
- 17Fluorescence quantum yields: ΦF,DCM=0.07–0.18 for alkylaminocorannulenes 4 a–h, ΦF,DCM=0.18–0.25 for arylaminocorannulenes 4 i,j. These quantum yields were significantly larger than that of the pristine corannulene (ΦF,DCM=0.013), especially in the case of arylaminocorannulenes.
- 18The fluorescence quantum yields of bis(alkylamino)corannulenes in the solid state were 0.02–0.09.
- 19The fluorescence spectrum of 9 in PMMA matrix was blue-shifted compared with that in solution. See supporting information for the details.
- 20H. Zhu, I. Badía-Domínguez, B. Shi, Q. Li, P. Wei, H. Xing, M. C. R. Delgado, F. Huang, J. Am. Chem. Soc. 2021, 143, 2164.
- 21
- 21aK. Schmidt, S. Brovelli, V. Coropceanu, J.-L. Brédas, C. Bazzini, T. Caronna, R. Tubino, F. Meinardi, J. Phys. Chem. A 2006, 110, 11018;
- 21bK. Schmidt, S. Brovelli, V. Coropceanu, D. Beljonne, J. Cornil, C. Bazzini, T. Caronna, R. Tubino, F. Meinardi, Z. Shuai, J.-L. Brédas, J. Phys. Chem. A 2007, 111, 10490.
- 22The reduction potential was consistent with the previously reported values though the measurement conditions were not the same with those in this work:
- 22aJ. Janata, J. Gendell, C.-Y. Ling, W. Barth, L. Backes, H. B. Mark, Jr., R. G. Lawton, J. Am. Chem. Soc. 1967, 89, 3056;
- 22bT. J. Seiders, K. K. Baldridge, J. S. Siegel, R. Gleiter, Tetrahedron Lett. 2000, 41, 4519;
- 22cC. Bruno, R. Benassi, A. Passalacqua, F. Paolucci, C. Fontanesi, M. Marcaccio, E. A. Jackson, L. T. Scott, J. Phys. Chem. B 2009, 113, 1954;
- 22dC. Bruno, E. Ussano, G. Barucca, D. Vanossi, G. Valenti, E. A. Jackson, A. Goldoni, L. Litti, S. Fermani, L. Pasquali, M. Meneghetti, C. Fontanesi, L. T. Scott, F. Paoluccia, M. Marcaccio, Chem. Sci. 2021, 12, 8048.
- 23Extended application of Cahn-Ingold-Prelog rule to corannulenes: M. A. Petrukhina, K. W. Andreini, L. Peng, L. T. Scott, Angew. Chem. Int. Ed. 2004, 43, 5477; Angew. Chem. 2004, 116, 5593.
- 24
- 24aJ. C. Hanson, C. E. Nordman, Acta Crystallogr. Sect. B 1976, 32, 1147;
- 24bM. A. Petrukhina, K. W. Andreini, J. Mack, L. T. Scott, J. Org. Chem. 2005, 70, 5713.
- 25Two bowl-depth values for the two crystallographically independent corannulene molecules are shown. Data source: ref. [23b]. Crystal detail: P21/c (No. 14), a=13.1560(8), b=11.6708(7), c=16.2931(9) Å, β=102.1740(10)°, Z=8, R1=0.0370.
- 26
- 26aC. Lee, W. Yang, R. G. Parr, Phys. Rev. B 1988, 37, 785;
- 26bA. D. Becke, J. Chem. Phys. 1993, 98, 1372.
- 27
- 27aA. Schäfer, H. Horn, R. Ahlrichs, J. Chem. Phys. 1992, 97, 2571;
- 27bF. Weigend, R. Ahlrichs, Phys. Chem. Chem. Phys. 2005, 7, 3297.
- 28
- 28aS. Grimme, J. Antony, S. Ehrlich, H. Krieg, J. Chem. Phys. 2010, 132, 154104;
- 28bS. Grimme, S. Ehrlich, L. Goerigk, J. Comput. Chem. 2011, 32, 1456.
- 29
- 29aP. George, M. Trachtman, C. W. Bock, A. M. Brett, Tetrahedron 1976, 32, 317;
- 29bV. I. Minkin, Pure Appl. Chem. 1999, 71, 1919.
- 30S. Maeda, Y. Harabuchi, Y. Sumiya, M. Takagi, K. Suzuki, M. Hatanaka, Y. Osada, T. Taketsugu, K. Morokuma, K. Ohno, GRRM17, see http://iqce.jp/GRRM/index_e.shtml (accessed date 14 Jul., 2021).
- 31
- 31aS. Maeda, K. Ohno, K. Morokuma, Phys. Chem. Chem. Phys. 2013, 15, 3683;
- 31bS. Maeda, Y. Harabuchi, M. Takagi, K. Saita, K. Suzuki, T. Ichino, Y. Sumiya, K. Sugiyama, Y. Ono, J. Comput. Chem. 2018, 39, 233.
- 32
- 32aS. Hayakawa, A. Kawasaki, Y. Hong, D. Uraguchi, T. Ooi, D. Kim, T. Akutagawa, N. Fukui, H. Shinokubo, J. Am. Chem. Soc. 2019, 141, 19807;
- 32bH. Murase, Y. Nagata, S. Akahori, H. Shinokubo, Y. Miyake, Chem. Asian J. 2020, 15, 3873.
- 33Some expected EQs such as 10-M,PMMMM (10-P,PPPPM) were unlocated not simply due to the error termination of the calculations but due to the undesired bowl inversion during the geometry optimization steps, which afford diastereomers with different chirality compared with the original input structures.
- 34Some conformers are connected by more than one pathway, but in Figure 5, only one of the pathways with lower activation energies is shown for clarity. For example, 10-M,PPMPM and 10-M,PPPPM are connected by two transition states: 10-TS-M,PPPPM-h-M,PPMPM (14.1 kcal mol−1) and 10-TS-M,PPPPM-h-M,PPMPM (12.6 kcal mol−1), depending on which helicene is involved in inversion. Here, the helical chirality of the helicene involving inversion is expressed in underlined P/M, and -h- means the two conformers are connected via helicene inversion. In this example, the latter TS has lower energy, so in Figure 5 a, only interconversion pathway through 10-TS-M,PPPPM-h-M,PPMPM is shown. For full representation of the interconversion networks, see Figure S58,61.
- 35In the 1H NMR spectrum of 11 at room temperature, the most upfield-shifted singlet signal around 3.7 ppm is assigned as the methyl protons in azahelicene D in 11-M,PMPMM (Table 2) and ones in the corresponding azahelicene in 11-P,PPMPM, according to the simulated 1H chemical shifts. See Table S27–30 and Figure S62 for detail.
- 36Deposition Number(s) 2109309 (8), 2109310 (9), 2109311 (10), 2109312 (14), 2109313(15), and 2109314 (16) contain(s) the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.