Photoinitiated anti-Hydropentafluorosulfanylation of Terminal Alkynes
Dr. Mélodie Birepinte
CCVC, PROTEO, Département de chimie, Université Laval, 1045 avenue de la Médecine, Québec, G1V 0A6 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Pier Alexandre Champagne
Department of Chemistry and Environmental Science, New Jersey Institute of Technology, Newark, NJ, 07102 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jean-François Paquin
CCVC, PROTEO, Département de chimie, Université Laval, 1045 avenue de la Médecine, Québec, G1V 0A6 Canada
Search for more papers by this authorDr. Mélodie Birepinte
CCVC, PROTEO, Département de chimie, Université Laval, 1045 avenue de la Médecine, Québec, G1V 0A6 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Pier Alexandre Champagne
Department of Chemistry and Environmental Science, New Jersey Institute of Technology, Newark, NJ, 07102 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jean-François Paquin
CCVC, PROTEO, Département de chimie, Université Laval, 1045 avenue de la Médecine, Québec, G1V 0A6 Canada
Search for more papers by this authorGraphical Abstract
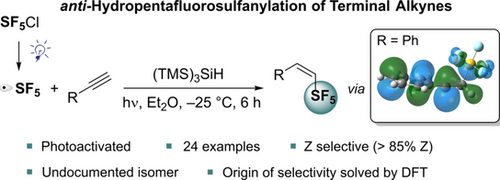
The direct synthesis of new (Z)-(1-alken-1-yl)pentafluoro-λ6-sulfanes was performed via the photoinitiated anti-hydropentafluorosulfanylation of terminal alkynes. The reaction of SF5Cl with 24 substrates in the presence of (TMS)3SiH as a H-atom donor gave access to the undocumented Z-isomer. The origins of this unusual selectivity were investigated by DFT calculations, which provide a better understanding of the geometry and reactivity of SF5-substituted vinylic radicals.
Abstract
A photoinitiated anti-hydropentafluorosulfanylation of terminal alkynes using SF5Cl and (TMS)3SiH as the hydrogen atom donor is reported. This transformation generates selectively (Z)-(1-alken-1-yl)pentafluoro-λ6-sulfanes (Z:E : >85:15), thus allowing the preparation of this previously unknown geometrical isomer. DFT calculations highlight that the selectivity is due to the intrinsic preference of SF5-substituted vinylic radicals to adopt a cis geometry, and to increased steric contacts during the transition structures leading to the minor (E)-products.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202112575-sup-0001-misc_information.pdf9.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. M. Thayer, Chem. Eng. News 2006, 84, 27–32.
- 2P. R. Savoie, J. T. Welch, Chem. Rev. 2015, 115, 1130–1190.
- 3For selected reviews/perspectives, see
- 3aS. Altomonte, M. Zanda, J. Fluorine Chem. 2012, 143, 57–93;
- 3bV. Gouverneur, K. Müller in Fluorine in Pharmaceutical and Medicinal Chemistry. From Biophysical Aspects to Clinical Applications, World Scientific Publishing, 2012, pp. 175–207;
- 3cN. A. Meanwell, J. Med. Chem. 2018, 61, 5822–5880.
- 4Review: J. M. W. Chan, J. Mater. Chem. C 2019, 7, 12822–12834.
- 5Selected reviews:
- 5aJ. Charpentier, N. Früh, A. Togni, Chem. Rev. 2015, 115, 650–682;
- 5bX. Liu, C. Xu, N. Wang, Q. Liu, Chem. Rev. 2015, 115, 683–730;
- 5cX. Yang, T. Wu, R. J. Phipps, F. D. Toste, Chem. Rev. 2015, 115, 826–870;
- 5dS. Barata-Vallejo, B. Lantaño, A. Postigo, Chem. Eur. J. 2014, 20, 16806–16829;
- 5eR. P. Bhaskaran, B. P. Babu, Adv. Synth. Catal. 2020, 362, 5219–5237;
- 5fH. Xiao, Z. Zhang, Y. Fang, L. Zhu, C. Li, Chem. Soc. Rev. 2021, 50, 6308–6319;
- 5gC. R. Pitts, D. Bornemann, P. Liebing, N. Santschi, A. Togni, Angew. Chem. Int. Ed. 2019, 58, 1950–1954; Angew. Chem. 2019, 131, 1970–1974;
- 5hI. Saidalimu, Y. Liang, K. Niina, K. Tanagawa, N. Saito, N. Shibata, Org. Chem. Front. 2019, 6, 1157–1161;
- 5iD. Rombach, H.-A. Wagenknecht, ChemCatChem 2018, 10, 2955–2961;
- 5jG. Iakobson, M. Pošta, P. Beier, J. Fluorine Chem. 2018, 213, 51–55;
- 5kD. Rombach, H.-A. Wagenknecht, Angew. Chem. Int. Ed. 2020, 59, 300–303; Angew. Chem. 2020, 132, 306–310;
- 5lD. Rombach, B. Birenheide, H.-A. Wagenknecht, Chem. Eur. J. 2021, 27, 8088–8093.
- 6For selected examples, see:
- 6aS. Mizuta, S. Verhoog, K. M. Engle, T. Khotavivattana, M. O'Duill, K. Wheelhouse, G. Rassias, M. Médebielle, V. Gouverneur, J. Am. Chem. Soc. 2013, 135, 2505–2508;
- 6bS. Choi, Y. J. Kim, S. M. Kim, J. W. Yang, S. W. Kim, E. J. Cho, Nat. Commun. 2014, 5, 4881;
- 6cY. Cheng, S. Yu, Org. Lett. 2016, 18, 2962–2965;
- 6dL. Zhu, L.-S. Wang, B. Li, B. Fu, C.-P. Zhang, W. Li, Chem. Commun. 2016, 52, 6371–6374;
- 6eL. He, X. Yang, G. C. Tsui, J. Org. Chem. 2017, 82, 6192–6201;
- 6fY.-Y. Ren, X. Zheng, X. Zhang, Synlett 2018, 29, 1029–1032;
- 6gA.-L. Barthelemy, G. Dagousset, E. Magnier, Eur. J. Org. Chem. 2020, 1429–1432.
- 7The hydro(chloro)pentafluorosulfanylation of diazo compounds has been recently described, see: J.-Y. Shou, X.-H. Xu, F.-L. Qing, Angew. Chem. Int. Ed. 2021, 60, 15271–15275; Angew. Chem. 2021, 133, 15399–15403.
- 8For applications in material chemistry, see for example:
- 8aP. Kirsch, J. T. Binder, E. Lork, G.-V. Röschenthaler, Fluorine Chem. 2006, 127, 610–619;
- 8bM. V. Ponomarenko, N. Kalinovich, Y. A. Serguchev, M. Bremer, G.-V. Röschenthaler, J. Fluorine Chem. 2012, 135, 68–74;
- 8cR. Winter, P. G. Nixon, G. L. Gard, D. J. Graham, D. G. Castner, N. R. Holcomb, D. W. Grainger, Langmuir 2004, 20, 5776–5781.
- 9For applications in bio-relevant molecules, see for example:
- 9aD. S. Lim, J.-H. Li, J. T. Welch, Eur. J. Org. Chem. 2012, 3946–3954;
- 9bE. Forcellini, S. Boutin, C.-A. Lefebvre, E. E. Shayhidin, M.-C. Boulanger, G. Rhéaume, X. Barbeau, P. Lagüe, P. Mathieu, J.-F. Paquin, Eur. J. Med. Chem. 2018, 147, 130–149;
- 9cV. K. Brel, O. I. Artyushin, A. A. Moiseeva, E. V. Sharova, A. G. Buyanovskaya, Y. V. Nelyubina, J. Sulfur Chem. 2020, 41, 29–43.
- 10For applications as synthetic intermediates for the preparation of more elaborated SF5-containing compounds, see for example:
- 10aV. K. Brel, Synthesis 2006, 2, 339–343;
- 10bV. K. Brel, Fluorine Chem. 2007, 128, 862–867;
- 10cR. W. Winter, G. L. Gard, J. Fluorine Chem. 2007, 128, 896–901;
- 10dW. S. Husstedt, J. S. Thrasher, G. Haufe, Synlett 2011, 1683–1686;
- 10eA.-L. Dreier, A. V. Matsnev, J. S. Thrasher, G. Haufe, J. Fluorine Chem. 2014, 167, 84–90;
- 10fE. Falkowska, V. Tognetti, L. Joubert, P. Jubault, J.-P. Bouillon, X. Pannecoucke, RSC Adv. 2015, 5, 6864–6868;
- 10gE. Falkowska, M. Y. Laurent, V. Tognetti, L. Joubert, P. Jubault, J.-P. Bouillon, X. Pannecoucke, Tetrahedron 2015, 71, 8067–8076;
- 10hJ. Desroches, E. Forcellini, J.-F. Paquin, Eur. J. Org. Chem. 2016, 4611–4620;
- 10iJ. Desroches, A. Gilbert, C. Houle, J.-F. Paquin, Synthesis 2017, 49, 4827–4844;
- 10jQ. Zhao, T. M. H. Vuong, X.-F. Bai, X. Pannecoucke, L.-W. Xu, J.-P. Bouillon, P. Jubault, Chem. Eur. J. 2018, 24, 5644–5651.
- 11The (E)-(1-alken-1-yl)pentafluoro-λ6-sulfanes are essentially prepared by the radical addition of SF5X (X=Cl or Br) onto an alkene followed by a base-promoted elimination. See ref. 2 for more details. A single report from 1983 mentions the preparation of a mixture of configurational isomers, but only the trans-product was characterized (as being the major one), see: J. Wessel, G. Kleemann, K. Seppelt, Chem. Ber. 1983, 116, 2399–2407.
- 12T. P. M. Goumans, K. van Alem, G. Lodder, Eur. J. Org. Chem. 2008, 435–443.
- 13B. Giese, Angew. Chem. Int. Ed. Engl. 1989, 28, 969–980; Angew. Chem. 1989, 101, 993–1004.
- 14
- 14aS. Aït-Mohand, W. R. Dolbier, Org. Lett. 2002, 4, 3013–3015;
- 14bW. R. Dolbier Jr, S. Aït-Mohand, T. D. Schertz, T. A. Sergeeva, J. A. Cradlebaugh, A. Mitani, G. L. Gard, R. W. Winter, J. S. Thrasher, J. Fluorine Chem. 2006, 127, 1302–1310.
- 15
- 15aA. Gilbert, P. Langowski, M. Delgado, L. Chabaud, M. Pucheault, J.-F. Paquin, Beilstein J. Org. Chem. 2020, 16, 3069–3077;
- 15bA. Gilbert, P. Langowski, M. Delgado, L. Chabaud, M. Pucheault, J.-F. Paquin, Beilstein J. Org. Chem. 2021, 17, 1725–1726;
- 15cA. Gilbert, M. Birepinte, J.-F. Paquin, J. Fluorine Chem. 2021, 243, 109734.
- 16For selected examples, see
- 16aJ. R. Case, N. H. Ray, H. L. Roberts, J. Chem. Soc. 1961, 2070–2075;
- 16bT. Grelbig, T. Krügerke, K. Z. Seppelt, Z. Anorg. Allg. Chem. 1987, 544, 74–80;
- 16cA. Klauck, K. Seppelt, Angew. Chem. Int. Ed. Engl. 1994, 33, 93–95; Angew. Chem. 1994, 106, 98–100;
- 16dI. V. Trushkov, V. K. Brel, Tetrahedron Lett. 2005, 46, 4777–4779;
- 16eV. K. Brel, Synthesis 2005, 8, 1245–1250;
- 16fV. K. Brel, Synthesis 2006, 2, 339–343;
- 16gM. V. Ponomarenko, Y. A. Serguchev, G.-V. Röschenthaler, J. Fluorine Chem. 2010, 131, 270–273.
- 17Though only the results using the 20 W blacklight CFL bulb (λmax=370 nm) are presented, the use of violet LEDs (λmax=400 nm) was equally effective. See the Supporting Information for the details.
- 18Review: C. Chatgilialoglu, Chem. Eur. J. 2008, 14, 2310–2320.
- 19See the Supporting Information for details.
- 20A. Gilbert, J.-F. Paquin, J. Fluorine Chem. 2019, 221, 70–74.
- 21A possible explanation for this observation could be a HAT from the SF5 radical and (TMS)3SiH. This reaction would generate sulfur fluoride hydride, SF5H, an unreported compound, as a transient species. The latter would either decompose to HF and SF4 or exist as a HF complex as suggested by theoretical studies, see:
- 21aK. O. Christe, D. A. Dixon, W. W. Wilson, J. Am. Chem. Soc. 1994, 116, 7123–7128;
- 21bQ. Li, L. Gong, Y. Xie, H. F. Schaefer, J. Am. Chem. Soc. 2004, 126, 14950–14959. This unproductive pathway would further consume SF5Cl, resulting in lower yields of 2 a. While we have not been able to observe SF5H, HF or SF4 by NMR, preliminary calculations indicate that the reaction of the SF5 radical with (TMS)3SiH is plausible with a similar reaction free energy to addition of the SF5 radical on the alkyne.
- 22The activation of pyridine-trans-tetrafluoro-λ6-sulfanyl chlorides in the presence or absence of light has been reported, see: K. Niina, K. Tanagawa, Y. Sumii, N. Saito, N. Shibata, Org. Chem. Front. 2020, 7, 1276–1282.
- 23
- 23aJ. H. Blackwell, R. Kumar, M. J. Gaunt, J. Am. Chem. Soc. 2021, 143, 1598–1609;
- 23bG. Kang, D. Romo, ACS Catal. 2021, 11, 1309–1315.
- 24
- 24aG. H. Lovett, S. Chen, X.-S. Xue, K. N. Houk, D. W. C. MacMillan, J. Am. Chem. Soc. 2019, 141, 20031–20036;
- 24bX.-M. Cao, Z.-R. Li, J.-B. Wang, X.-Y. Li, Theor. Chem. Acc. 2020, 139, 94;
- 24cJ. W. Lee, S. Lim, D. N. Maienshein, P. Liu, M.-Y. Ngai, Angew. Chem. Int. Ed. 2020, 59, 21475–21480; Angew. Chem. 2020, 132, 21659–21664.
- 25C. Galli, A. Guarnieri, H. Koch, P. Mencarelli, Z. Rappoport, J. Org. Chem. 1997, 62, 4072–4077.
- 26We estimate the S−Cl bond energy in SF5Cl at 60.6 kcal mol−1, making its homolytic cleavage likely.
- 27With 1 d, addition of the CF3 radical is exergonic by 27.7 kcal mol−1.