Accessing Unsymmetrically Linked Heterocycles through Stereoselective Palladium-Catalyzed Domino Cyclization
Ramon Arora
Davenport Research Laboratories, Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, M5S 3H6 Canada
Search for more papers by this authorDr. José F. Rodríguez
Davenport Research Laboratories, Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, M5S 3H6 Canada
Search for more papers by this authorDr. Andrew Whyte
Davenport Research Laboratories, Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, M5S 3H6 Canada
Search for more papers by this authorCorresponding Author
Prof. Mark Lautens
Davenport Research Laboratories, Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, M5S 3H6 Canada
Search for more papers by this authorRamon Arora
Davenport Research Laboratories, Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, M5S 3H6 Canada
Search for more papers by this authorDr. José F. Rodríguez
Davenport Research Laboratories, Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, M5S 3H6 Canada
Search for more papers by this authorDr. Andrew Whyte
Davenport Research Laboratories, Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, M5S 3H6 Canada
Search for more papers by this authorCorresponding Author
Prof. Mark Lautens
Davenport Research Laboratories, Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, M5S 3H6 Canada
Search for more papers by this authorGraphical Abstract
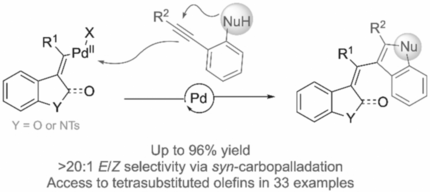
Solving alkynes of problems with palladium. A palladium-catalyzed domino cyclization of alkyne-tethered carbamoyl chlorides and aryl iodides with alkyne-tethered aryl nucleophiles is reported. This methodology unsymmetrically links heterocycles along a tetrasubstituted olefin with >20:1 E/Z selectivity and up to 96 % yield.
Abstract
A palladium-catalyzed strategy is presented to synthesize unsymmetrically linked heterocycles within stereoselective tetrasubstituted olefins. This reaction is proposed to occur via a vinyl-PdII intermediate capable of initiating the cyclization of various alkyne-tethered nucleophiles. Products are formed in up to 96 % yield and excellent stereoselectivities are obtained using low catalyst loadings. This transformation was scalable up to 1 mmol and mechanistic studies suggest a syn-carbopalladation of the carbamoyl chloride followed by PdII-catalyzed cyclization of alkyne-tethered nucleophiles.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202112288-sup-0001-misc_information.pdf6.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Muthukumar, G. N. Rao, G. Sekar, Org. Biomol. Chem. 2019, 17, 3921–3933;
- 1bK. Yuan, L. Liu, J. Chen, S. Guo, H. Yao, A. Lin, Org. Lett. 2018, 20, 3477–3481;
- 1cS. H. Siadatifard, M. Abdoli-Senejani, M. A. Bodaghifard, Cogent Chem. 2016, 2, 1188435;
- 1dN. Chadha, O. Silakari, Eur. J. Med. Chem. 2017, 134, 159–184;
- 1eY. M. Khetmalis, M. Shivani, S. Murugesan, K. V. G. Chandra Sekhar, Biomed. Pharmacother. 2021, 141, 111842.
- 2
- 2aF. McKoy, J. Chen, T. Schupbach, M. H. Hecht, Chem. Biol. Drug Des. 2014, 84, 505–512;
- 2bR. E. Staub, B. Onisko, L. F. Bjeldanes, Chem. Res. Toxicol. 2006, 19, 436–442;
- 2cR. Singh, E. S. Masuda, D. G. Payan, J. Med. Chem. 2012, 55, 3614–3643;
- 2dS.-S. Ma, W.-L. Mei, Z.-K. Guo, S.-B. Liu, Y.-X. Zhao, D.-L. Yang, Y.-B. Zeng, B. Jiang, H.-F. Dai, Org. Lett. 2013, 15, 1492–1495.
- 3
- 3aL. Mo, Z. Ma, Z. Zhang, Synth. Commun. 2005, 35, 1997–2004;
- 3bK. Kamata, M. Kiyota, A. Naoe, S. Nakatani, Y. Yamamoto, M. Hayashi, K. Komiyama, T. Yamori, M. Ishibashi, Chem. Pharm. Bull. 2005, 53, 594–597;
- 3cH. Banari, H. Kiyani, A. Pourali, Res. Chem. Intermed. 2017, 43, 1635–1649;
- 3dN. Azizi, E. Gholibeghlo, Z. Manocheri, Sci. Iran. 2012, 19, 574–578;
- 3eM. Marrelli, X. Cachet, F. Conforti, R. Sirianni, A. Chimento, V. Pezzi, S. Michel, G. A. Statti, F. Menichini, Nat. Prod. Res. 2013, 27, 2039–2045;
- 3fC. Chen, B. Hong, W. Li, T. Chang, G. Lee, Asian J. Org. Chem. 2017, 6, 426–431.
- 4C.-L. Ren, T. Zhang, X.-Y. Wang, T. Wu, J. Ma, Q.-Q. Xuan, F. Wei, H.-Y. Huang, D. Wang, L. Liu, Org. Biomol. Chem. 2014, 12, 9881–9886.
- 5G. Zhu, G. Bao, Y. Li, W. Sun, J. Li, L. Hong, R. Wang, Angew. Chem. Int. Ed. 2017, 56, 5332–5335; Angew. Chem. 2017, 129, 5416–5419.
- 6
- 6aH. A. Döndaş, M. D. G. Retamosa, J. M. Sansano, Organometallics 2019, 38, 1828–1867;
- 6bM. Pérez-Gómez, J. A. García-López, Angew. Chem. Int. Ed. 2016, 55, 14389–14393; Angew. Chem. 2016, 128, 14601–14605;
- 6cR. Grigg, V. Sridharan, J. Organomet. Chem. 1999, 576, 65–87;
- 6dM. Pérez-Gómez, S. Hernández-Ponte, D. Bautista, J. A. García-López, Chem. Commun. 2017, 53, 2842–2845.
- 7
- 7aA. De Meijere, P. Von Zezschwitz, S. Eräse, Acc. Chem. Res. 2005, 38, 413–422;
- 7bK. R. Holman, A. M. Stanko, S. E. Reisman, Chem. Soc. Rev. 2021, 50, 7891–7908;
- 7cR. Grigg, V. Loganathan, V. Sridharan, Tetrahedron Lett. 1996, 37, 3399–3402;
- 7dA. Pinto, Y. Jia, L. Neuville, J. Zhu, Chem. Eur. J. 2007, 13, 961–967;
- 7eH. Zheng, Y. Zhu, Y. Shi, Angew. Chem. Int. Ed. 2014, 53, 11280–11284; Angew. Chem. 2014, 126, 11462–11466;
- 7fT. Piou, L. Neuville, J. Zhu, Angew. Chem. Int. Ed. 2012, 51, 11561–11565; Angew. Chem. 2012, 124, 11729–11733;
- 7gD. C. Wang, H. X. Wang, E. J. Hao, X. H. Jiang, M. S. Xie, G. R. Qu, H. M. Guo, Adv. Synth. Catal. 2016, 358, 494–499;
- 7hY. Gao, Y. Gao, W. Wu, H. Jiang, X. Yang, W. Liu, C. J. Li, Chem. Eur. J. 2017, 23, 793–797;
- 7iR. Grigg, P. Fretwell, C. Meerholtz, V. Sridharan, Tetrahedron 1994, 50, 359–370;
- 7jI. Franzoni, H. Yoon, J. A. García-López, A. I. Poblador-Bahamonde, M. Lautens, Chem. Sci. 2018, 9, 1496–1509;
- 7kH. Yoon, M. Rölz, F. Landau, M. Lautens, Angew. Chem. Int. Ed. 2017, 56, 10920–10923; Angew. Chem. 2017, 129, 11060–11063;
- 7lH. Yoon, Y. J. Jang, M. Lautens, Synthesis 2016, 48, 1483–1490;
- 7mJ. Bajohr, A. G. Diallo, A. Whyte, S. Gaillard, J. L. Renaud, M. Lautens, Org. Lett. 2021, 23, 2797–2801;
- 7nA. Lu, X. Ji, B. Zhou, Z. Wu, Y. Zhang, Angew. Chem. Int. Ed. 2018, 57, 3233–3237; Angew. Chem. 2018, 130, 3287–3291;
- 7oT. Yao, T. Liu, C. Zhang, Chem. Commun. 2017, 53, 2386–2389;
- 7pC. Shao, Z. Wu, X. Ji, B. Zhou, Y. Zhang, Chem. Commun. 2017, 53, 10429–10432;
- 7qC. Chen, L. Liu, W. Sun, B. Zhu, ChemistrySelect 2021, 6, 6464–6467.
- 8
- 8aB. Yao, C. Jaccoud, Q. Wang, J. Zhu, Chem. Eur. J. 2012, 18, 5864–5868;
- 8bB. Yao, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2013, 52, 12992–12996; Angew. Chem. 2013, 125, 13230–13234;
- 8cV. Nori, A. Arcadi, A. Carlone, F. Marinelli, M. Chiarini, Beilstein J. Org. Chem. 2020, 16, 1084–1091.
- 9A. Whyte, J. Bajohr, R. Arora, A. Torelli, M. Lautens, Angew. Chem. Int. Ed. 2021, 60, 20231–20236; Angew. Chem. 2021, 133, 20393–20398.
- 10
- 10aW. S. Cheung, R. J. Patch, M. R. Player, J. Org. Chem. 2005, 70, 3741–3744;
- 10bS. Park, J. Lee, K. J. Shin, E. Oh, J. H. Seo, R. A. Bunce, Molecules 2017, 22, 503;
- 10cC. G. Dong, P. Yeung, Q. S. Hu, Org. Lett. 2007, 9, 363–366;
- 10dP. Wagner, M. Gulea, J. Suffert, M. Donnard, Chem. Eur. J. 2017, 23, 7458–7462.
- 11
- 11aA. Siva Reddy, A. L. Siva Kumari, S. Saha, K. C. Kumara Swamy, Adv. Synth. Catal. 2016, 358, 1625–1638;
- 11bS. Tang, P. Peng, S.-F. Pi, Y. Liang, N.-X. Wang, J.-H. Li, Org. Lett. 2008, 10, 1179–1182.
- 12C. Cheng, Y. Zhang, Org. Lett. 2021, 23, 5772–5776.
- 13R. Yanada, S. Obika, T. Inokuma, K. Yanada, M. Yamashita, S. Ohta, Y. Takemoto, J. Org. Chem. 2005, 70, 6972–6975.
- 14
- 14aU. K. Sharma, N. Sharma, Y. Kumar, B. K. Singh, E. V. Van Der Eycken, Chem. Eur. J. 2016, 22, 481–485;
- 14bX. X. Wu, A. Liu, M. Mou, H. Chen, S. Chen, J. Org. Chem. 2018, 83, 14181–14194.
- 15
- 15aC. M. Le, T. Sperger, R. Fu, X. Hou, Y. H. Lim, F. Schoenebeck, M. Lautens, J. Am. Chem. Soc. 2016, 138, 14441–14448;
- 15bC. M. Le, X. Hou, T. Sperger, F. Schoenebeck, M. Lautens, Angew. Chem. Int. Ed. 2015, 54, 15897–15900; Angew. Chem. 2015, 127, 16127–16131;
- 15cT. Sperger, C. M. Le, M. Lautens, F. Schoenebeck, Chem. Sci. 2017, 8, 2914–2922;
- 15dJ. Wang, Z. Dong, C. Yang, G. Dong, Nat. Chem. 2019, 11, 1106–1112;
- 15eA. B. Flynn, W. W. Ogilvie, Chem. Rev. 2007, 107, 4698–4745;
- 15fB. X. Li, D. N. Le, K. A. Mack, A. McClory, N. K. Lim, T. Cravillion, S. Savage, C. Han, D. B. Collum, H. Zhang, F. Gosselin, J. Am. Chem. Soc. 2017, 139, 10777–10783.
- 16
- 16aG. Cantagrel, B. de Carné-Carnavalet, C. Meyer, J. Cossy, Org. Lett. 2009, 11, 4262–4265;
- 16bJ. F. Rodríguez, A. Zhang, J. Bajohr, A. Whyte, B. Mirabi, M. Lautens, Angew. Chem. Int. Ed. 2021, 60, 18478–18483; Angew. Chem. 2021, 133, 18626–18631.
- 17Deposition Numbers 2094547 (for 3a), 2117158 (for 3r), 2094549 (for 5a), and 2094550 (for 5e) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.