Rhodium-Catalyzed Atroposelective Access to Axially Chiral Olefins via C−H Bond Activation and Directing Group Migration
Ruijie Mi
School of Chemistry and Chemical Engineering, Shaanxi Normal University (SNNU), Xi'an, 710062 China
Search for more papers by this authorHaohua Chen
School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 400030 China
Search for more papers by this authorDr. Xukai Zhou
Department of Chemistry, The University of Chicago, Chicago, IL, 60637 USA
Search for more papers by this authorProf. Dr. Nan Li
School of Chemistry and Chemical Engineering, Shaanxi Normal University (SNNU), Xi'an, 710062 China
Search for more papers by this authorDanqing Ji
School of Chemistry and Chemical Engineering, Shaanxi Normal University (SNNU), Xi'an, 710062 China
Search for more papers by this authorCorresponding Author
Dr. Fen Wang
School of Chemistry and Chemical Engineering, Shaanxi Normal University (SNNU), Xi'an, 710062 China
Search for more papers by this authorProf. Dr. Yu Lan
School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 400030 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xingwei Li
School of Chemistry and Chemical Engineering, Shaanxi Normal University (SNNU), Xi'an, 710062 China
Institute of Molecular Science and Engineering, Institute of Frontier and Interdisciplinary Sciences, Shandong University, Qingdao, 266237 China
Search for more papers by this authorRuijie Mi
School of Chemistry and Chemical Engineering, Shaanxi Normal University (SNNU), Xi'an, 710062 China
Search for more papers by this authorHaohua Chen
School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 400030 China
Search for more papers by this authorDr. Xukai Zhou
Department of Chemistry, The University of Chicago, Chicago, IL, 60637 USA
Search for more papers by this authorProf. Dr. Nan Li
School of Chemistry and Chemical Engineering, Shaanxi Normal University (SNNU), Xi'an, 710062 China
Search for more papers by this authorDanqing Ji
School of Chemistry and Chemical Engineering, Shaanxi Normal University (SNNU), Xi'an, 710062 China
Search for more papers by this authorCorresponding Author
Dr. Fen Wang
School of Chemistry and Chemical Engineering, Shaanxi Normal University (SNNU), Xi'an, 710062 China
Search for more papers by this authorProf. Dr. Yu Lan
School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 400030 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xingwei Li
School of Chemistry and Chemical Engineering, Shaanxi Normal University (SNNU), Xi'an, 710062 China
Institute of Molecular Science and Engineering, Institute of Frontier and Interdisciplinary Sciences, Shandong University, Qingdao, 266237 China
Search for more papers by this authorGraphical Abstract
Abstract
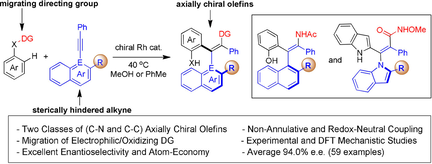
Axially chiral open-chain olefins represent an underexplored class of chiral platform. In this report, two classes of tetrasubstituted axially chiral acyclic olefins have been accessed in excellent enantioselectivity and regioselectivity via C−H activation of (hetero)arenes assisted by a migratable directing group en route to coupling with sterically hindered alkynes. The coupling of indoles bearing an N-aminocarbonyl directing group afforded C–N axially chiral acrylamides with the assistance of a racemic zinc carboxylate additive. DFT studies suggest a β-nitrogen elimination–reinsertion pathway for the directing group migration. Meanwhile, the employment of N-phenoxycarboxamide delivered C−C axially chiral enamides via migration of the oxidizing directing group. Experiments suggest that in both cases the (hetero)arene substrate adopts a well-defined orientation during the C−H activation, which in turn determines the disposition of the alkyne in migratory insertion. Synthetic applications of representative chiral olefins are demonstrated.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202111860-sup-0001-misc_information.pdf10.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. J. Walsh, M. C. Kozlowski, Fundamentals of Asymmetric Catalysis, University Science Books, Washington, 2009;
- 1bG. Ma, M. P. Sibi, Chem. Eur. J. 2015, 21, 11644;
- 1cJ. Wencel-Delord, A. Panossian, F. R. Leroux, F. Colobert, Chem. Soc. Rev. 2015, 44, 3418.
- 2For selected reviews, see:
- 2aG. Bringmann, A. J. P. Mortimer, P. A. Keller, M. J. Gresser, J. Garner, M. Breuning, Angew. Chem. Int. Ed. 2005, 44, 5384; Angew. Chem. 2005, 117, 5518;
- 2bM. C. Kozlowski, B. J. Morgan, E. C. Linton, Chem. Soc. Rev. 2009, 38, 3193;
- 2cG. Bringmann, T. Gulder, T. A. M. Gulder, M. Breuning, Chem. Rev. 2011, 111, 563;
- 2dE. Kumarasamy, R. Raghunathan, M. P. Sibi, J. Sivaguru, Chem. Rev. 2015, 115, 11239;
- 2eP. Renzi, Org. Biomol. Chem. 2017, 15, 4506;
- 2fY.-B. Wang, B. Tan, Acc. Chem. Res. 2018, 51, 534;
- 2gJ. K. Cheng, S.-H. Xiang, S. Li, L. Ye, B. Tan, Chem. Rev. 2021, 121, 4805.
- 3
- 3aO. Baudoin, Eur. J. Org. Chem. 2005, 4223;
- 3bK. Tanaka, Chem. Asian J. 2009, 4, 508;
- 3cA. Link, C. Sparr, Chem. Soc. Rev. 2018, 47, 3804.
- 4
- 4aR. Adams, M. W. Miller, J. Am. Chem. Soc. 1940, 62, 53.
- 5
- 5aR. Adams, A. W. Anderson, M. W. Miller, J. Am. Chem. Soc. 1941, 63, 1589;
- 5bR. Adams, J. W. Mecorney, J. Am. Chem. Soc. 1945, 67, 798;
- 5cR. W. Baker, T. W. Hambley, P. Turner, B. J. Wallace, Chem. Commun. 1996, 2571;
- 5dT. Hattori, M. Date, K. Sakurai, N. Morohashi, H. Kosugi, S. Miyano, Tetrahedron Lett. 2001, 42, 8035;
- 5eJ. Feng, Z. Gu, SynOpen 2021, 5, 68.
- 6S.-C. Zheng, S. Wu, Q. Zhou, L. W. Chung, L. Ye, B. Tan, Nat. Commun. 2017, 8, 15238.
- 7
- 7aS. Jia, Z. Chen, N. Zhang, Y. Tan, Y. Liu, J. Deng, H.-L. Yan, J. Am. Chem. Soc. 2018, 140, 7056;
- 7bY. Tan, S.-Q. Jia, F.-L. Hu, Y.-D. Liu, L. Peng, D.-M. Li, H.-L. Yan, J. Am. Chem. Soc. 2018, 140, 16893;
- 7cS. Li, D. Xu, F. Hu, D. Li, W. Qin, H. Yan, Org. Lett. 2018, 20, 7665;
- 7dD. Li, Y. Tan, L. Peng, S. Li, N. Zhang, Y. Liu, H. Yan, Org. Lett. 2018, 20, 4959;
- 7eA. Huang, L. Zhang, D. Li, Y. Liu, H. Yan, W. Li, Org. Lett. 2019, 21, 95.
- 8Y. Liang, J. Ji, X. Zhang, Q. Jiang, J. Luo, X. Zhao, Angew. Chem. Int. Ed. 2020, 59, 4959; Angew. Chem. 2020, 132, 4989.
- 9
- 9aY. B. Wang, P. Yu, Z.-P. Zhou, J. Zhang, J. Wang, S.-H. Luo, Q.-S. Gu, K. N. Houk, B. Tan, Nat. Catal. 2019, 2, 504;
- 9bC. Ma, F.-T. Sheng, H.-Q. Wang, S. Deng, Y.-C. Zhang, Y. Jiao, W. Tan, F. Shi, J. Am. Chem. Soc. 2020, 142, 15686;
- 9cC. S. Wang, T. Z. Li, S. J. Liu, Y. C. Zhang, S. Deng, Y. Jiao, F. Shi, Chin. J. Chem. 2020, 38, 543.
- 10J. Feng, B. Li, Y. He, Z. Gu, Angew. Chem. Int. Ed. 2016, 55, 2186; Angew. Chem. 2016, 128, 2226.
- 11
- 11aG. Liao, T. Zhou, Q. J. Yao, B.-F. Shi, Chem. Commun. 2019, 55, 8514;
- 11bC.-X. Liu, W.-W. Zhang, S.-Y. Yin, Q. Gu, S.-L. You, J. Am. Chem. Soc. 2021, 143, 14025.
- 12
- 12aL. Jin, Q.-J. Yao, P.-P. Xie, N. Li, B.-B. Zhan, Y.-Q. Han, X. Hong, B.-F. Shi, Chem 2020, 6, 497;
- 12bH. Song, Y. Li, Q.-J. Yao, L. Jin, L. Liu, Y.-H. Liu, B.-F. Shi, Angew. Chem. Int. Ed. 2020, 59, 6576; Angew. Chem. 2020, 132, 6638.
- 13C. Yang, T.-R. Wu, Y. Li, B.-B. Wu, R.-X. Jin, D.-D. Hu, Y.-B. Li, K.-J. Bian, X.-S. Wang, Chem. Sci. 2021, 12, 3726.
- 14
- 14aJ. Wang, X. Qi, X.-L. Min, W. Yi, P. Liu, Y. He, J. Am. Chem. Soc. 2021, 143, 10686;
- 14bQ.-R. Zhao, R. Jiang, S.-L. You, Acta Chim. Sin. 2021, 79, 1107.
- 15Selected reviews:
- 15aH. M. L. Davies, R. E. J. Beckwith, Chem. Rev. 2003, 103, 2861;
- 15bM. P. Doyle, R. Duffy, M. Ratnikov, L. Zhou, Chem. Rev. 2010, 110, 704;
- 15cK. M. Engle, J. Q. Yu, J. Org. Chem. 2013, 78, 8927;
- 15dJ. Wencel-Delord, F. Colobert, Chem. Eur. J. 2013, 19, 14010;
- 15eF. Zhang, D. R. Spring, Chem. Soc. Rev. 2014, 43, 6906;
- 15fG. Song, X. Li, Acc. Chem. Res. 2015, 48, 1007;
- 15gZ. Chen, B. Wang, J. Zhang, W. Yu, Z. Liu, Y. Zhang, Org. Chem. Front. 2015, 2, 1107;
- 15hJ. He, M. Wasa, K. S. L. Chan, Q. Shao, J.-Q. Yu, Chem. Rev. 2017, 117, 8754;
- 15iT. Gensch, M. N. Hopkinson, F. Glorius, Chem. Soc. Rev. 2016, 45, 2900;
- 15jJ. R. Hummel, J. A. Boerth, J. A. Ellman, Chem. Rev. 2017, 117, 9163;
- 15kZ. Dong, Z. Ren, S. J. Thompson, Y. Xu, G. Dong, Chem. Rev. 2017, 117, 9333;
- 15lY. Park, Y. Kim, S. Chang, Chem. Rev. 2017, 117, 9247;
- 15mT. G. Saint-Denis, R. Y. Zhu, G. Chen, Q. F. Wu, J.-Q. Yu, Science 2018, 359, 4798;
- 15nJ. Diesel, N. Cramer, ACS Catal. 2019, 9, 9164;
- 15oJ. Loup, U. Dhawa, F. Pesciaioli, J. Wencel-Delord, L. Ackermann, Angew. Chem. Int. Ed. 2019, 58, 12803; Angew. Chem. 2019, 131, 12934;
- 15pŁ. Woźniak, N. Cramer, Trends Chem. 2019, 1, 471;
- 15qP. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz, L. Ackermann, Chem. Rev. 2019, 119, 2192;
- 15rS. Rej, N. Chatani, Angew. Chem. Int. Ed. 2019, 58, 8304; Angew. Chem. 2019, 131, 8390;
- 15sQ. Shao, K. Wu, Z. Zhang, S. Q. Qian, J.-Q. Yu, Acc. Chem. Res. 2020, 53, 833.
- 16
- 16aF. Wang, Z. Qi, Y. Zhao, S. Zhai, G. Zheng, R. Mi, Z. Huang, X. Zhu, X. He, X. Li, Angew. Chem. Int. Ed. 2020, 59, 13288; Angew. Chem. 2020, 132, 13390;
- 16bF. Wang, J. Jing, Y. Zhao, X. Zhu, X.-P. Zhang, L. Zhao, P. Hu, W.-Q. Deng, X. Li, Angew. Chem. Int. Ed. 2021, 60, 16628; Angew. Chem. 2021, 133, 16764.
- 17Z.-J. Fang, S.-C. Zheng, Z. Guo, J.-Y. Guo, B. Tan, X.-Y. Liu, Angew. Chem. Int. Ed. 2015, 54, 9528; Angew. Chem. 2015, 127, 9664.
- 18Y. Wu, C. Pi, Y. Wu, X. Cui, Chem. Soc. Rev. 2021, 50, 3677.
- 19
- 19aG. Liu, Y. Shen, Z. Zhou, X. Lu, Angew. Chem. Int. Ed. 2013, 52, 6033; Angew. Chem. 2013, 125, 6149;
- 19bX. Zhang, Z. Qi, X. Li, Angew. Chem. Int. Ed. 2014, 53, 10794; Angew. Chem. 2014, 126, 10970;
- 19cT. Piou, T. Rovis, Nature 2015, 527, 86;
- 19dZ.-Y. Hu, X.-F. Tong, G.-X. Liu, Org. Lett. 2016, 18, 1702;
- 19eX. Wang, T. Gensch, A. Lerchen, C. G. Daniliuc, F. Glorius, J. Am. Chem. Soc. 2017, 139, 6506;
- 19fY.-X. Wu, Z.-Q. Chen, Y.-X. Yang, W.-L. Zhu, B. Zhou, J. Am. Chem. Soc. 2018, 140, 42.
- 20
- 20aX. Wu, Y. Lu, J. Qiao, W. Dai, X. Jia, H. Ni, X. Zhang, H. Liu, F. Zhao, Org. Lett. 2020, 22, 9163;
- 20bF. Zhao, X. Gong, Y. Lu, J. Qiao, X. Jia, H. Ni, X. Wu, X. Zhang, Org. Lett. 2021, 23, 727;
- 20cX. Xu, L. Zhang, H. Zhao, Y. Pan, J. Li, Z. Luo, J. Han, L. Xu, M. Lei, Org. Lett. 2021, 23, 4624;
- 20dJ. Zhang, H. Xie, H. Zhu, S. Zhang, M. R. Lonka, H. Zou, ACS Catal. 2019, 9, 10233.
- 21
- 21aH. Ikemoto, T. Yoshino, K. Sakata, S. Matsunaga, M. Kanai, J. Am. Chem. Soc. 2014, 136, 5424;
- 21bH. Ikemoto, R. Tanaka, K. Sakata, M. Kanai, T. Yoshino, S. Matsunaga, Angew. Chem. Int. Ed. 2017, 56, 7156; Angew. Chem. 2017, 129, 7262;
- 21cC. Zhu, R. Kuniyil, B. B. Jei, L. Ackermann, ACS Catal. 2020, 10, 4444;
- 21dA. Lerchen, T. Knecht, C. G. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 2016, 55, 15166; Angew. Chem. 2016, 128, 15391;
- 21eK. Sakata, M. Eda, Y. Kitaoka, T. Yoshino, S. Matsunaga, J. Org. Chem. 2017, 82, 7379.
- 22
- 22aC.-M. Wang, A. Wang, M. Rueping, Angew. Chem. Int. Ed. 2017, 56, 9935; Angew. Chem. 2017, 129, 10067;
- 22bS.-Y. Chen, X.-L. Han, J.-Q. Wu, Q.-J. Li, Y.-Y. Chen, H.-G. Wang, Angew. Chem. Int. Ed. 2017, 56, 9939; Angew. Chem. 2017, 129, 10071.
- 23
- 23aC. Duchemin, N. Cramer, Angew. Chem. Int. Ed. 2020, 59, 14129; Angew. Chem. 2020, 132, 14233;
- 23bK. Ozols, S. Onodera, Ł. Woźniak, N. Cramer, Angew. Chem. Int. Ed. 2021, 60, 655; Angew. Chem. 2021, 133, 665.
- 24L. Wu, H. Xu, H. Gao, L. Li, W. Chen, Z. Zhou, W. Yi, ACS Catal. 2021, 11, 2279.
- 25
- 25aB. Ye, N. Cramer, J. Am. Chem. Soc. 2013, 135, 636;
- 25bB. Ye, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 7896; Angew. Chem. 2014, 126, 8030.
- 26
- 26aJ. Zheng, S.-L. You, Angew. Chem. Int. Ed. 2014, 53, 13244; Angew. Chem. 2014, 126, 13460;
- 26bZ.-J. Jia, C. Merten, R. Gontla, C. G. Daniliuc, A. P. Antonchick, H. Waldmann, Angew. Chem. Int. Ed. 2017, 56, 2429; Angew. Chem. 2017, 129, 2469;
- 26cY.-S. Jang, Ł. Woźniak, J. Pedroni, N. Cramer, Angew. Chem. Int. Ed. 2018, 57, 12901; Angew. Chem. 2018, 130, 13083;
- 26dH. Li, X. Yan, J. Zhang, W. Guo, J. Jiang, J. Wang, Angew. Chem. Int. Ed. 2019, 58, 6732; Angew. Chem. 2019, 131, 6804;
- 26eM. Tian, D. Bai, G. Zheng, J. Chang, X. Li, J. Am. Chem. Soc. 2019, 141, 9527;
- 26fQ. Wang, W.-W. Zhang, H. Song, J. Wang, C. Zheng, Q. Gu, S.-L. You, J. Am. Chem. Soc. 2020, 142, 15678;
- 26gC. Pan, S.-Y. Yin, S.-B. Wang, Q. Gu, S.-L. You, Angew. Chem. Int. Ed. 2021, 60, 15510; Angew. Chem. 2021, 133, 15638;
- 26hL. Sun, H. Chen, B. Liu, J. Chang, L. Kong, F. Wang, Y. Lan, X. Li, Angew. Chem. Int. Ed. 2021, 60, 8391; Angew. Chem. 2021, 133, 8472;
- 26iJ. Wang, H. Chen, L. Kong, F. Wang, Y. Lan, X. Li, ACS Catal. 2021, 11, 9151.
- 27Reviews on asymmetric C−H bond activation:
- 27aJ. Mas-Roselló, A. G. Herraiz, B. Audic, A. Laverny, N. Cramer, Angew. Chem. Int. Ed. 2021, 60, 13198; Angew. Chem. 2021, 133, 13306;
- 27bŁ. Woźniak, J.-F. Tan, Q.-H. Nguyen, A. M. du Vigné, V. Smal, Y.-X. Cao, N. Cramer, Chem. Rev. 2020, 120, 10516;
- 27cT. K. Achar, S. Maiti, S. Jana, D. Maiti, ACS Catal. 2020, 10, 13748;
- 27dC. G. Newton, S.-G. Wang, C. C. Oliveira, N. Cramer, Chem. Rev. 2017, 117, 8908;
- 27eB. Ye, N. Cramer, Acc. Chem. Res. 2015, 48, 1308;
- 27fT. Yoshino, S. Matsunaga, ACS Catal. 2021, 11, 6455;
- 27gQ. Gu, Z.-J. Wu, S.-L. You, Bull. Chem. Soc. Jpn. 2021, 94, 641.
- 28Y. Jang, M. Dieckmann, N. Cramer, Angew. Chem. Int. Ed. 2017, 56, 15088; Angew. Chem. 2017, 129, 15284.
- 29B. Shen, B. Wan, X. Li, Angew. Chem. Int. Ed. 2018, 57, 15534; Angew. Chem. 2018, 130, 15760.
- 30J. Sun, W. Yuan, R. Tian, P. Wang, X.-P. Zhang, X. Li, Angew. Chem. Int. Ed. 2020, 59, 22706; Angew. Chem. 2020, 132, 22895.
- 31M. Moselage, J. Li, F. Kramm, L. Ackermann, Angew. Chem. Int. Ed. 2017, 56, 5341; Angew. Chem. 2017, 129, 5425.