Enantioselective Synthesis of Chiral Carboxylic Acids from Alkynes and Formic Acid by Nickel-Catalyzed Cascade Reactions: Facile Synthesis of Profens
Dr. Peng Yang
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorYaxin Sun
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorKaiyue Fu
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
Search for more papers by this authorLi Zhang
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
Search for more papers by this authorGuang Yang
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
Search for more papers by this authorJieyu Yue
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
Search for more papers by this authorCorresponding Author
Dr. Yu Ma
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jianrong Steve Zhou
State Key Laboratory of Chemical Oncogenomics, Key Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Room F312, 2199 Lishui Road, Nanshan District, Shenzhen, 518055 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Bo Tang
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
Search for more papers by this authorDr. Peng Yang
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorYaxin Sun
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorKaiyue Fu
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
Search for more papers by this authorLi Zhang
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
Search for more papers by this authorGuang Yang
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
Search for more papers by this authorJieyu Yue
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
Search for more papers by this authorCorresponding Author
Dr. Yu Ma
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jianrong Steve Zhou
State Key Laboratory of Chemical Oncogenomics, Key Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Room F312, 2199 Lishui Road, Nanshan District, Shenzhen, 518055 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Bo Tang
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for, Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014 P. R. China
Search for more papers by this authorGraphical Abstract
Abstract
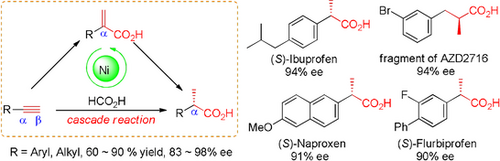
We report a stereoselective conversion of terminal alkynes to α-chiral carboxylic acids using a nickel-catalyzed domino hydrocarboxylation-transfer hydrogenation reaction. A simple nickel/BenzP* catalyst displayed high activity in both steps of regioselective hydrocarboxylation of alkynes and subsequent asymmetric transfer hydrogenation. The reaction was successfully applied in enantioselective preparation of three nonsteroidal anti-inflammatory profens (>90 % ees) and the chiral fragment of AZD2716.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202111778-sup-0001-exp_399.cif127.8 KB | Supporting Information |
| anie202111778-sup-0001-misc_information.pdf12 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. Lamberth, J. Dinges, Bioactive Carboxylic Compound Classes: Pharmaceuticals and Agrochemicals, Wiley-VCH, 2016;
10.1002/9783527693931 Google Scholar
- 1bA. Halama, M. Zapadlo, Org. Process Res. Dev. 2019, 23, 102–107.
- 2
- 2aH. Alper, N. Hamel, J. Am. Chem. Soc. 1990, 112, 2803–2804;
- 2bM. D. Miquel-Serrano, A. Aghmiz, M. Diéguez, A. M. Masdeu-Bultó, C. Claver, D. Sinou, Tetrahedron: Asymmetry 1999, 10, 4463–4467;
- 2cT. M. Konrad, J. A. Fuentes, A. M. Z. Slawin, M. L. Clarke, Angew. Chem. Int. Ed. 2010, 49, 9197–9200; Angew. Chem. 2010, 122, 9383–9386;
- 2dT. M. Konrad, J. T. Durrani, C. J. Cobley, M. L. Clarke, Chem. Commun. 2013, 49, 3306–3308;
- 2eG. J. Harkness, M. L. Clarke, Eur. J. Org. Chem. 2017, 4859–4863;
- 2fS. Feng, S. L. Buchwald, J. Am. Chem. Soc. 2021, 143, 4935–4941;
- 2gD. Tian, R. Xu, J. Zhu, J. Huang, W. Dong, J. Claverie, W. Tang, Angew. Chem. Int. Ed. 2021, 60, 6305–6309; Angew. Chem. 2021, 133, 6375–6379;
- 2hY.-H. Yao, X.-J. Zou, Y. Wang, H.-Y. Yang, Z.-H. Ren, Z.-H. Guan, Angew. Chem. Int. Ed. 2021, 60, 23117–23122.
- 3
- 3aT. Hayashi, N. Kawamura, Y. Ito, J. Am. Chem. Soc. 1987, 109, 7876–7878;
- 3bT. Ohta, H. Takaya, M. Kitamura, K. Nagai, R. Noyori, J. Org. Chem. 1987, 52, 3174–3176;
- 3cT. Uemura, X. Zhang, K. Matsumura, N. Sayo, H. Kumobayashi, T. Ohta, K. Nozaki, H. Takaya, J. Org. Chem. 1996, 61, 5510–5516;
- 3dR. Hoen, J. A. F. Boogers, H. Bernsmann, A. J. Minnaard, A. Meetsma, T. D. Tiemersma-Wegman, A. H. M. de Vries, J. G. de Vries, B. L. Feringa, Angew. Chem. Int. Ed. 2005, 44, 4209–4212; Angew. Chem. 2005, 117, 4281–4284;
- 3eW. Chen, P. J. McCormack, K. Mohammed, W. Mbafor, S. M. Roberts, J. Whittall, Angew. Chem. Int. Ed. 2007, 46, 4141–4144; Angew. Chem. 2007, 119, 4219–4222;
- 3fY. Zhang, Z. Han, F. Li, K. Ding, A. Zhang, Chem. Commun. 2010, 46, 156–158;
- 3gS.-F. Zhu, Y.-B. Yu, S. Li, L.-X. Wang, Q.-L. Zhou, Angew. Chem. Int. Ed. 2012, 51, 8872–8875; Angew. Chem. 2012, 124, 9002–9005;
- 3hY. Li, K. Dong, Z. Wang, K. Ding, Angew. Chem. Int. Ed. 2013, 52, 6748–6752; Angew. Chem. 2013, 125, 6880–6884;
- 3iC. Chen, H. Wang, Z. Zhang, S. Jin, S. Wen, J. Ji, L. W. Chung, X.-Q. Dong, X. Zhang, Chem. Sci. 2016, 7, 6669–6673;
- 3jJ. Li, J. Shen, C. Xia, Y. Wang, D. Liu, W. Zhang, Org. Lett. 2016, 18, 2122–2125;
- 3kQ. Yan, D. Kong, W. Zhao, G. Zi, G. Hou, J. Org. Chem. 2016, 81, 2070–2077;
- 3lC. Chen, S. Wen, X.-Q. Dong, X. Zhang, Org. Chem. Front. 2017, 4, 2034–2038;
- 3mQ. Wang, Z. Zhang, C. Chen, H. Yang, Z. Han, X.-Q. Dong, X. Zhang, Org. Chem. Front. 2017, 4, 627–630;
- 3nS.-F. Zhu, Q.-L. Zhou, Acc. Chem. Res. 2017, 50, 988–1001;
- 3oX. Du, Y. Xiao, J.-M. Huang, Y. Zhang, Y.-N. Duan, H. Wang, C. Shi, G.-Q. Chen, X. Zhang, Nat. Commun. 2020, 11, 3239;
- 3pX. Liu, S. Liu, Q. Wang, G. Zhou, L. Yao, Q. Ouyang, R. Jiang, Y. Lan, W. Chen, Org. Lett. 2020, 22, 3149–3154;
- 3qH. Zhong, M. Shevlin, P. J. Chirik, J. Am. Chem. Soc. 2020, 142, 5272–5281;
- 3rX. Du, Y. Xiao, Y. Yang, Y.-N. Duan, F. Li, Q. Hu, L. W. Chung, G.-Q. Chen, X. Zhang, Angew. Chem. Int. Ed. 2021, 60, 11384–11390; Angew. Chem. 2021, 133, 11485–11491;
- 3sM. Shevlin, M. R. Friedfeld, H. Sheng, N. A. Pierson, J. M. Hoyt, L.-C. Campeau, P. J. Chirik, J. Am. Chem. Soc. 2016, 138, 3562–3569.
- 4C. An, M. H. Shaw, A. Tharp, D. Verma, H. Li, H. Wang, X. Wang, Org. Lett. 2020, 22, 8320–8325.
- 5S. Zhang, H. Neumann, M. Beller, Chem. Soc. Rev. 2020, 49, 3187–3210.
- 6
- 6aM. Aoki, M. Kaneko, S. Izumi, K. Ukai, N. Iwasawa, Chem. Commun. 2004, 2568–2569;
- 6bT. Fujihara, T. Xu, K. Semba, J. Terao, Y. Tsuji, Angew. Chem. Int. Ed. 2011, 50, 523–527; Angew. Chem. 2011, 123, 543–547;
- 6cS. Li, W. Yuan, S. Ma, Angew. Chem. Int. Ed. 2011, 50, 2578–2582; Angew. Chem. 2011, 123, 2626–2630;
- 6dX. Wang, Y. N. Lim, C. Lee, H.-Y. Jang, B. Y. Lee, Eur. J. Org. Chem. 2013, 1867–1871;
- 6eX. Wang, M. Nakajima, R. Martin, J. Am. Chem. Soc. 2015, 137, 8924–8927;
- 6fL. Feng, X. Li, B. Liu, E. Vessally, J. CO2 Util. 2020, 40, 101220;
- 6gW. Xiong, F. Shi, R. Cheng, B. Zhu, L. Wang, P. Chen, H. Lou, W. Wu, C. Qi, M. Lei, H. Jiang, ACS Catal. 2020, 10, 7968–7978;
- 6hM.-M. Wang, S.-M. Lu, K. Paridala, C. Li, Chem. Commun. 2021, 57, 1230–1233.
- 7
- 7aM. Periasamy, U. Radhakrishman, C. Rameshkumar, J.-J. Brunet, Tetrahedron Lett. 1997, 38, 1623–1626;
- 7bM. Periasamy, C. Rameshkumar, U. Radhakrishnan, J.-J. Brunet, J. Org. Chem. 1998, 63, 4930–4935;
- 7cM. Beesu, M. Periasamy, J. Organomet. Chem. 2012, 705, 30–33;
- 7dJ.-B. Peng, F.-P. Wu, X.-F. Wu, Chem. Rev. 2019, 119, 2090–2127.
- 8
- 8aD. Zargarian, H. Alper, Organometallics 1993, 12, 712–724;
- 8bJ. Hou, J.-H. Xie, Q.-L. Zhou, Angew. Chem. Int. Ed. 2015, 54, 6302–6305; Angew. Chem. 2015, 127, 6400–6403;
- 8cM.-C. Fu, R. Shang, W.-M. Cheng, Y. Fu, ACS Catal. 2016, 6, 2501–2505;
- 8dJ. Hou, M.-L. Yuan, J.-H. Xie, Q.-L. Zhou, Green Chem. 2016, 18, 2981–2984;
- 8eJ. Jiang, M. Fu, C. Li, R. Shang, Y. Fu, Organometallics 2017, 36, 2818–2825.
- 9
- 9aP. Yang, H. Xu, J. Zhou, Angew. Chem. Int. Ed. 2014, 53, 12210–12213; Angew. Chem. 2014, 126, 12406–12409;
- 9bS. Guo, P. Yang, J. Zhou, Chem. Commun. 2015, 51, 12115–12117;
- 9cH. Xu, P. Yang, P. Chuanprasit, H. Hirao, J. Zhou, Angew. Chem. Int. Ed. 2015, 54, 5112–5116; Angew. Chem. 2015, 127, 5201–5205;
- 9dP. Yang, L. H. Lim, P. Chuanprasit, H. Hirao, J. Zhou, Angew. Chem. Int. Ed. 2016, 55, 12083–12087; Angew. Chem. 2016, 128, 12262–12266.
- 10Examples of converting alkynes to carboxylic acids by one-pot or sequential reactions:
- 10aJ.-T. Lee, H. Alper, Tetrahedron Lett. 1991, 32, 1769–1770;
- 10bI. A. Nozomi Saito, K. Hayashi, K. Hamada, M. Koyama, Y. Sato, Org. Biomol. Chem. 2016, 14, 10080–10089;
- 10cD. Yu, F. Zhou, D. S. W. Lim, H. Su, Y. Zhang, ChemSusChem 2017, 10, 836–841.
- 11
- 11aK. Tamura, M. Sugiya, K. Yoshida, A. Yanagisawa, T. Imamoto, Org. Lett. 2010, 12, 4400–4403;
- 11bT. Imamoto, K. Tamura, Z. Zhang, Y. Horiuchi, M. Sugiya, K. Yoshida, A. Yanagisawa, I. D. Gridnev, J. Am. Chem. Soc. 2012, 134, 1754–1769.
- 12
- 12aL. Cassar, J. Organomet. Chem. 1975, 93, 253–257;
- 12bH. A. Dieck, F. R. Heck, J. Organomet. Chem. 1975, 93, 259–263;
- 12cK. Sonogashira, Y. Tohda, N. Hagihara, Tetrahedron Lett. 1975, 16, 4467–4470.
- 13
- 13aL. Wu, Q. Liu, R. Jackstell, M. Beller, Angew. Chem. Int. Ed. 2014, 53, 6310–6320; Angew. Chem. 2014, 126, 6426–6436;
- 13bY. Wang, W. Ren, Y. Shi, Org. Biomol. Chem. 2015, 13, 8416–8419;
- 13cW. Ren, W. Chang, J. Dai, Y. Shi, J. Li, Y. Shi, J. Am. Chem. Soc. 2016, 138, 14864–14867;
- 13dW. Liu, W. Ren, J. Li, Y. Shi, W. Chang, Y. Shi, Org. Lett. 2017, 19, 1748–1751;
- 13eX. Lv, F. Huang, Y.-B. Wu, G. Lu, Catal. Sci. Technol. 2018, 8, 2835–2840;
- 13fR. Sang, P. Kucmierczyk, K. Dong, R. Franke, H. Neumann, R. Jackstell, M. Beller, J. Am. Chem. Soc. 2018, 140, 5217–5223;
- 13gY.-N. Wu, M.-C. Fu, R. Shang, Y. Fu, Chem. Commun. 2020, 56, 4067–4069.