A Biomimetic Approach for Spatially Controlled Cell Membrane Engineering Using Fusogenic Spherical Nucleic Acid
Minjie Lin
Institute of Chemical Biology and Nanomedicine, State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan Provincial Key Laboratory of Biomacromolecular Chemical Biology, College of Chemistry and Chemical Engineering, Hunan University, Changsha, 410082 China
These authors contributed equally to this work.
Search for more papers by this authorYuanyuan Chen
Institute of Chemical Biology and Nanomedicine, State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan Provincial Key Laboratory of Biomacromolecular Chemical Biology, College of Chemistry and Chemical Engineering, Hunan University, Changsha, 410082 China
These authors contributed equally to this work.
Search for more papers by this authorSisi Zhao
Institute of Chemical Biology and Nanomedicine, College of Biology, Hunan University, Changsha, 410082 China
These authors contributed equally to this work.
Search for more papers by this authorRui Tang
Institute of Chemical Biology and Nanomedicine, State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan Provincial Key Laboratory of Biomacromolecular Chemical Biology, College of Chemistry and Chemical Engineering, Hunan University, Changsha, 410082 China
Search for more papers by this authorProf. Dr. Zhou Nie
Institute of Chemical Biology and Nanomedicine, State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan Provincial Key Laboratory of Biomacromolecular Chemical Biology, College of Chemistry and Chemical Engineering, Hunan University, Changsha, 410082 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hang Xing
Institute of Chemical Biology and Nanomedicine, State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan Provincial Key Laboratory of Biomacromolecular Chemical Biology, College of Chemistry and Chemical Engineering, Hunan University, Changsha, 410082 China
Search for more papers by this authorMinjie Lin
Institute of Chemical Biology and Nanomedicine, State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan Provincial Key Laboratory of Biomacromolecular Chemical Biology, College of Chemistry and Chemical Engineering, Hunan University, Changsha, 410082 China
These authors contributed equally to this work.
Search for more papers by this authorYuanyuan Chen
Institute of Chemical Biology and Nanomedicine, State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan Provincial Key Laboratory of Biomacromolecular Chemical Biology, College of Chemistry and Chemical Engineering, Hunan University, Changsha, 410082 China
These authors contributed equally to this work.
Search for more papers by this authorSisi Zhao
Institute of Chemical Biology and Nanomedicine, College of Biology, Hunan University, Changsha, 410082 China
These authors contributed equally to this work.
Search for more papers by this authorRui Tang
Institute of Chemical Biology and Nanomedicine, State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan Provincial Key Laboratory of Biomacromolecular Chemical Biology, College of Chemistry and Chemical Engineering, Hunan University, Changsha, 410082 China
Search for more papers by this authorProf. Dr. Zhou Nie
Institute of Chemical Biology and Nanomedicine, State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan Provincial Key Laboratory of Biomacromolecular Chemical Biology, College of Chemistry and Chemical Engineering, Hunan University, Changsha, 410082 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hang Xing
Institute of Chemical Biology and Nanomedicine, State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan Provincial Key Laboratory of Biomacromolecular Chemical Biology, College of Chemistry and Chemical Engineering, Hunan University, Changsha, 410082 China
Search for more papers by this authorGraphical Abstract
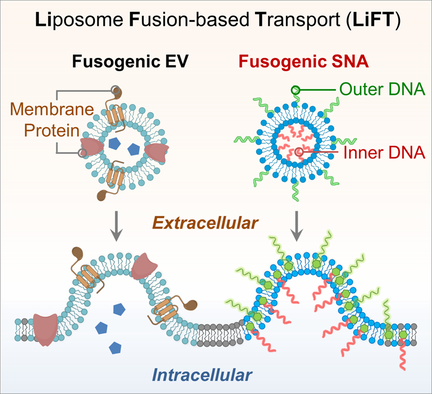
Inspired by the natural extracellular vesicle-cell fusion process, we introduced the fusogenic spherical nucleic acid (fuso-SNA) to achieve spatially controlled cell membrane engineering with DNA by using a liposome fusion-based transport (LiFT) approach. We demonstrated asymmetric DNA modifications on the cell membrane with orthogonal functionalities on each side, realizing applications such as heterotypic cell assembly and intracellular metabolites detection.
Abstract
Engineering of the cell plasma membrane using functional DNA is important for studying and controlling cellular behaviors. However, most efforts to apply artificial DNA interactions on cells are limited to external membrane surface due to the lack of suitable synthetic tools to engineer the intracellular side, which impedes many applications in cell biology. Inspired by the natural extracellular vesicle-cell fusion process, we have developed a fusogenic spherical nucleic acid construct to realize robust DNA functionalization on both external and internal cell surfaces via liposome fusion-based transport (LiFT) strategy, which enables applications including the construction of heterotypic cell assembly for programmed signaling pathway and detection of intracellular metabolites. This approach can engineer cell membranes in a highly efficient and spatially controlled manner, allowing one to build anisotropic membrane structures with two orthogonal DNA functionalities.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202111647-sup-0001-misc_information.pdf3.3 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH. E. Grecco, M. Schmick, P. I. H. Bastiaens, Cell 2011, 144, 897–909;
- 1bF. M. Goñi, Biochim. Biophys. Acta Biomembr. 2014, 1838, 1467–1476.
- 2
- 2aS. Toda, L. R. Blauch, S. K. Y. Tang, L. Morsut, W. A. Lim, Science 2018, 361, 156–162;
- 2bC. M. Csizmar, J. R. Petersburg, C. R. Wagner, Cell Chem. Biol. 2018, 25, 931–940.
- 3
- 3aS. Abbina, E. M. J. Siren, H. Moon, J. N. Kizhakkedathu, ACS Biomater. Sci. Eng. 2018, 4, 3658–3677;
- 3bJ. Fang, Y. Y. Hsueh, J. Soto, W. J. Sun, J. Q. Wang, Z. Gu, A. Khademhosseini, S. Li, ACS Nano 2020, 14, 1296–1318.
- 4
- 4aJ. W. Liu, Z. H. Cao, Y. Lu, Chem. Rev. 2009, 109, 1948–1998;
- 4bO. I. Wilner, I. Willner, Chem. Rev. 2012, 112, 2528–2556;
- 4cM. R. Jones, N. C. Seeman, C. A. Mirkin, Science 2015, 347, DOI: 10.1126/science.126091;
- 4dF. Hong, F. Zhang, Y. Liu, H. Yan, Chem. Rev. 2017, 117, 12584–12640;
- 4eD. Zhao, Y. H. Kong, S. S. Zhao, H. Xing, Top. Curr. Chem. 2020, 378, 83–124.
- 5
- 5aZ. J. Gartner, C. R. Bertozzi, Proc. Natl. Acad. Sci. USA 2009, 106, 4606–4610;
- 5bM. Langecker, V. Arnaut, J. List, F. C. Simmel, Acc. Chem. Res. 2014, 47, 1807–1815;
- 5cL. Y. Feng, J. Li, J. L. Sun, L. H. Wang, C. H. Fan, J. L. Shen, Adv. Healthcare Mater. 2021, 10, 2001718.
- 6X. Xiong, H. Liu, Z. Zhao, M. B. Altman, D. Lopez-Colon, C. J. Yang, L. J. Chang, C. Liu, W. Tan, Angew. Chem. Int. Ed. 2013, 52, 1472–1476; Angew. Chem. 2013, 125, 1512–1516.
- 7
- 7aJ. Yun, A. N. Cho, S. W. Cho, Y. S. Nam, Biomater. Sci. 2018, 6, 3388–3396;
- 7bR. C. Qian, Z. R. Zhou, W. J. Guo, Y. T. Wu, Z. L. Yang, Y. Lu, J. Am. Chem. Soc. 2021, 143, 5737–5744.
- 8
- 8aM. X. You, Y. F. Lyu, D. Han, L. P. Qiu, Q. L. Liu, T. Chen, C. S. Wu, L. Peng, L. Q. Zhang, G. Bao, W. H. Tan, Nat. Nanotechnol. 2017, 12, 453–459;
- 8bH. Li, M. Wang, T. H. Shi, S. H. Yang, J. H. Zhang, H. H. Wang, Z. Nie, Angew. Chem. Int. Ed. 2018, 57, 10226–10230; Angew. Chem. 2018, 130, 10383–10387.
- 9
- 9aW. A. Zhao, S. Schafer, J. Choi, Y. J. Yamanaka, M. L. Lombardi, S. Bose, A. L. Carlson, J. A. Phillips, W. S. Teo, I. A. Droujinine, C. H. Cui, R. K. Jain, J. Lammerding, J. C. Love, C. P. Lin, D. Sarkar, R. Karnik, J. M. Karp, Nat. Nanotechnol. 2011, 6, 524–531;
- 9bT. Tokunaga, S. Namiki, K. Yamada, T. Imaishi, H. Nonaka, K. Hirose, S. Sando, J. Am. Chem. Soc. 2012, 134, 9561–9564.
- 10
- 10aJ. R. Burns, N. Al-Juffali, S. M. Janes, S. Howorka, Angew. Chem. Int. Ed. 2014, 53, 12466–12470; Angew. Chem. 2014, 126, 12674–12678;
- 10bE. Akbari, M. Y. Mollica, C. R. Lucas, S. M. Bushman, R. A. Patton, M. Shahhosseini, J. W. Song, C. E. Castro, Adv. Mater. 2017, 29, 1703632.
- 11M. E. Todhunter, N. Y. Jee, A. J. Hughes, M. C. Coyle, A. Cerchiari, J. Farlow, J. C. Garbe, M. A. LaBarge, T. A. Desai, Z. J. Gartner, Nat. Methods 2015, 12, 975–981.
- 12
- 12aN. S. Selden, M. E. Todhunter, N. Y. Jee, J. S. Liu, K. E. Broaders, Z. J. Gartner, J. Am. Chem. Soc. 2012, 134, 765–768;
- 12bP. Shi, N. Zhao, J. P. Lai, J. Coyne, E. R. Gaddes, Y. Wang, Angew. Chem. Int. Ed. 2018, 57, 6800–6804; Angew. Chem. 2018, 130, 6916–6920;
- 12cT. Gao, T. S. Chen, C. Feng, X. He, C. L. Mu, J. Anzai, G. X. Li, Nat. Commun. 2019, 10, 2946;
- 12dP. Shi, Y. Wang, Angew. Chem. Int. Ed. 2021, 60, 11580–11591; Angew. Chem. 2021, 133, 11684–11695.
- 13
- 13aV. R. Martins, M. S. Dias, P. Hainaut, Curr. Opin. Oncol. 2013, 25, 66–75;
- 13bB. Podbilewicz, Annu. Rev. Cell Dev. Biol. 2014, 30, 111–139;
- 13cI. Prada, J. Meldolesi, Int. J. Mol. Sci. 2016, 17, 1296.
- 14
- 14aA. Montecalvo, A. T. Larregina, W. J. Shufesky, D. B. Stolz, M. L. G. Sullivan, J. M. Karlsson, C. J. Baty, G. A. Gibson, G. Erdos, Z. L. Wang, J. Milosevic, O. A. Tkacheva, S. J. Divito, R. Jordan, J. Lyons-Weiler, S. C. Watkins, A. E. Morelli, Blood 2012, 119, 756–766;
- 14bS. Ohno, A. Ishikawa, M. Kuroda, Adv. Drug Delivery Rev. 2013, 65, 398–401;
- 14cW. A. He, F. Calore, P. Londhe, A. Canella, D. C. Guttridge, C. M. Croce, Proc. Natl. Acad. Sci. USA 2014, 111, 4525–4529;
- 14dJ. Lee, H. Lee, U. Goh, J. Kim, M. Jeong, J. Lee, J. H. Park, ACS Appl. Mater. Interfaces 2016, 8, 6790–6795.
- 15
- 15aR. J. Banga, N. Chernyak, S. P. Narayan, S. T. Nguyen, C. A. Mirkin, J. Am. Chem. Soc. 2014, 136, 9866–9869;
- 15bC. E. Callmann, C. D. Kusmierz, J. W. Dittmar, L. Broger, C. A. Mirkin, ACS Cent. Sci. 2021, 7, 892–899.
- 16
- 16aA. Csiszár, N. Hersch, S. Dieluweit, R. Biehl, R. Merkel, B. Hoffmann, Bioconjugate Chem. 2010, 21, 537–543;
- 16bY. Onuki, Y. Obata, K. Kawano, H. Sano, R. Matsumoto, Y. Hayashi, K. Takayama, Mol. Pharm. 2016, 13, 369–378.
- 17Z. I. Imam, L. E. Kenyon, G. Ashby, F. Nagib, M. Mendicino, C. Zhao, A. K. Gadok, J. C. Stachowiak, Cell. Mol. Bioeng. 2017, 10, 387–403.
- 18D. Dutta, A. Pulsipher, W. Luo, M. N. Yousaf, J. Am. Chem. Soc. 2011, 133, 8704–8713.
- 19
- 19aY. Xu, F. C. Szoka, Jr., Biochemistry 1996, 35, 5616–5623;
- 19bH. Xing, L. Tang, X. J. Yang, K. Hwang, W. D. Wang, Q. Yin, N. Y. Wong, L. W. Dobrucki, N. Yasui, J. A. Katzenellenbogen, W. G. Helferich, J. J. Cheng, Y. Lu, J. Mater. Chem. B 2013, 1, 5288–5297;
- 19cA. J. Sprangers, L. L. Hao, R. J. Banga, C. A. Mirkin, Small 2017, 13, 1602753.
- 20N. Dave, J. W. Liu, ACS Nano 2011, 5, 1304–1312.
- 21K. Sakai-Kato, K. Yoshida, K. Izutsu, Chem. Phys. Lipids 2019, 224, 104726.
- 22J. Yan, Y. L. Tan, M. J. Lin, H. Xing, J. H. Jiang, Chem. Sci. 2021, 12, 1803–1809.
- 23H. Li, B. H. Zhang, X. G. Lu, X. Y. Tan, F. Jia, Y. Xiao, Z. H. Cheng, Y. Li, D. O. Silva, H. S. Schrekker, K. Zhang, C. A. Mirkin, Proc. Natl. Acad. Sci. USA 2018, 115, 4340–4344.
- 24
- 24aA. F. Radovic-Moreno, N. Chernyak, C. C. Mader, S. Nallagatla, R. S. Kang, L. L. Hao, D. A. Walker, T. L. Halo, T. J. Merkel, C. H. Rische, S. Anantatmula, M. Burkhart, C. A. Mirkin, S. M. Gryaznov, Proc. Natl. Acad. Sci. USA 2015, 112, 3892–3897;
- 24bS. Y. Wang, L. Qin, G. Yamankurt, K. Skakuj, Z. Y. Huang, P. C. Chen, D. Dominguez, A. Lee, B. Zhang, C. A. Mirkin, Proc. Natl. Acad. Sci. USA 2019, 116, 10473–10481.
- 25N. J. Agard, J. A. Prescher, C. R. Bertozzi, J. Am. Chem. Soc. 2004, 126, 15046–15047.
- 26
- 26aK. Y. Xun, K. Pei, X. J. Liu, X. Y. Peng, Y. L. Du, L. P. Qiu, W. H. Tan, J. Am. Chem. Soc. 2019, 141, 18013–18020;
- 26bZ. L. Ge, J. B. Liu, L. J. Guo, G. B. Yao, Q. Li, L. H. Wang, J. Li, C. H. Fan, J. Am. Chem. Soc. 2020, 142, 8800–8808;
- 26cM. S. Xiao, W. Lai, H. Z. Yu, Z. J. Yu, L. Li, C. H. Fan, H. Pei, J. Am. Chem. Soc. 2021, 143, 3448–3454.
- 27
- 27aW. T. Bellamy, Semin. Oncol. 2001, 28, 551–559;
- 27bR. Paduch, A. Walter-Croneck, B. Zdzisinska, A. Szuster-Ciesielska, M. Kandefer-Szerszen, Cell Biol. Int. 2005, 29, 497–505;
- 27cJ. L. Leight, D. L. Alge, A. J. Maier, K. S. Anseth, Biomaterials 2013, 34, 7344–7352;
- 27dL. Lin, X. X. Lin, L. Y. Lin, Q. Feng, T. Kitamori, J. M. Lin, J. S. Sun, Anal. Chem. 2017, 89, 10037–10044.
- 28
- 28aJ. Zhao, J. H. Gao, W. T. Xue, Z. H. Di, H. Xing, Y. Lu, L. L. Li, J. Am. Chem. Soc. 2018, 140, 578–581;
- 28bY. Bai, H. Xing, Y. Bai, L. H. Tan, K. Hwang, J. Li, Y. Lu, S. C. Zimmerman, Chem. Sci. 2020, 11, 1564–1572.
- 29Z. Di, J. Zhao, H. Chu, W. Xue, Y. Zhao, L. Li, Adv. Mater. 2019, 31, e1901885.