Structural and Molecular Basis of the Catalytic Mechanism of Geranyl Pyrophosphate C6-Methyltransferase: Creation of an Unprecedented Farnesyl Pyrophosphate C6-Methyltransferase
Graphical Abstract
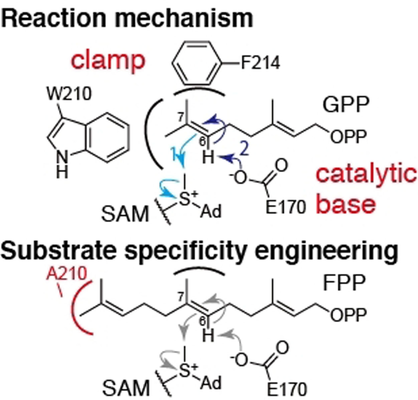
The structures of a geranyl pyrophosphate (GPP) C6-methyltransferase, BezA, and its complex with S-adenosyl homocysteine were solved by X-ray crystallography. Further analysis based on site-directed mutagenesis and computational calculations provided the molecular basis of the methylation reaction. Furthermore, an unprecedented farnesyl pyrophosphate C6-methyltransferase was created by introducing a W210A substitution.
Abstract
Prenyl pyrophosphate methyltransferases enhance the structural diversity of terpenoids. However, the molecular basis of their catalytic mechanisms is poorly understood. In this study, using multiple strategies, we characterized a geranyl pyrophosphate (GPP) C6-methyltransferase, BezA. Biochemical analysis revealed that BezA requires Mg2+ and solely methylates GPP. The crystal structures of BezA and its complex with S-adenosyl homocysteine were solved at 2.10 and 2.56 Å, respectively. Further analyses using site-directed mutagenesis, molecular docking, molecular dynamics simulations, and quantum mechanics/molecular mechanics calculations revealed the molecular basis of the methylation reaction. Importantly, the function of E170 as a catalytic base to complete the methylation reaction was established. We also succeeded in switching the substrate specificity by introducing a W210A substitution, resulting in an unprecedented farnesyl pyrophosphate C6-methyltransferase.
Conflict of interest
The authors declare no conflict of interest.